Alcohol-Induced Blood-Brain Barrier Impairment: An In Vitro Study
- PMID: 33799986
- PMCID: PMC7967408
- DOI: 10.3390/ijerph18052683
Alcohol-Induced Blood-Brain Barrier Impairment: An In Vitro Study
Abstract
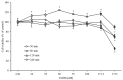
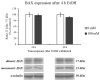
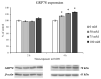
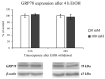
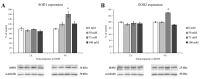
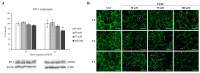
In recent years, alcohol abuse has dramatically grown with deleterious consequence for people's health and, in turn, for health care costs. It has been demonstrated, in humans and animals, that alcohol intoxication induces neuroinflammation and neurodegeneration thus leading to brain impairments. Furthermore, it has been shown that alcohol consumption is able to impair the blood-brain barrier (BBB), but the molecular mechanisms underlining this detrimental effect have not been fully elucidated. For this reason, in this study we investigated the effects of alcohol exposure on a rat brain endothelial (RBE4) cell line, as an in vitro-validated model of brain microvascular endothelial cells. To assess whether alcohol caused a concentration-related response, the cells were treated at different times with increasing concentrations (10-1713 mM) of ethyl alcohol (EtOH). Microscopic and molecular techniques, such as cell viability assay, immunofluorescence and Western blotting, were used to examine the mechanisms involved in alcohol-induced brain endothelial cell alterations including tight junction distribution, apoptosis, and reactive oxygen species production. Our findings clearly demonstrate that alcohol causes the formation of gaps between cells by tight junction disassembly, triggered by the endoplasmic reticulum and oxidative stress, highlighted by GRP78 chaperone upregulation and increase in reactive oxygen species production, respectively. The results from this study shed light on the mechanisms underlying alcohol-induced blood-brain barrier dysfunction and a better understanding of these processes will allow us to take advantage of developing new therapeutic strategies in order to prevent the deleterious effects of alcohol.
Keywords: alcohol abuse; alcoholism; blood–brain barrier; oxidative stress; tight junction.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- World Health Organization . Global Status Report on Alcohol and Health 2018. World Health Organization; Geneva, Switzerland: 2018. Management of Substance Abuse Team.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

