Platelet Phenotyping and Function Testing in Thrombocytopenia
- PMID: 33800006
- PMCID: PMC7962106
- DOI: 10.3390/jcm10051114
Platelet Phenotyping and Function Testing in Thrombocytopenia
Abstract
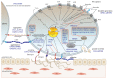
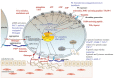
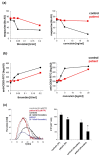
Patients who suffer from inherited or acquired thrombocytopenia can be also affected by platelet function defects, which potentially increase the risk of severe and life-threatening bleeding complications. A plethora of tests and assays for platelet phenotyping and function analysis are available, which are, in part, feasible in clinical practice due to adequate point-of-care qualities. However, most of them are time-consuming, require experienced and skilled personnel for platelet handling and processing, and are therefore well-established only in specialized laboratories. This review summarizes major indications, methods/assays for platelet phenotyping, and in vitro function testing in blood samples with reduced platelet count in relation to their clinical practicability. In addition, the diagnostic significance, difficulties, and challenges of selected tests to evaluate the hemostatic capacity and specific defects of platelets with reduced number are addressed.
Keywords: bleeding; flow cytometry; platelet count; platelet disorders; platelet function tests; thrombocytopenia.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Diagnosis of Platelet Function Disorders: A Challenge for Laboratories.Hamostaseologie. 2022 Feb;42(1):36-45. doi: 10.1055/a-1700-7036. Epub 2022 Feb 23. Hamostaseologie. 2022. PMID: 35196730 Review.
-
Platelet function tests: a comparative review.Vasc Health Risk Manag. 2015 Feb 18;11:133-48. doi: 10.2147/VHRM.S44469. eCollection 2015. Vasc Health Risk Manag. 2015. PMID: 25733843 Free PMC article. Review.
-
The role of platelets in bleeding in patients with thrombocytopenia and hematological disease.Clin Chem Lab Med. 2019 Nov 26;57(12):1808-1817. doi: 10.1515/cclm-2019-0380. Clin Chem Lab Med. 2019. PMID: 31465290 Review.
-
Perioperative monitoring of primary and secondary hemostasis in coronary artery bypass grafting.Semin Thromb Hemost. 2005;31(4):426-40. doi: 10.1055/s-2005-916678. Semin Thromb Hemost. 2005. PMID: 16149021
-
Critical issues in hematology: anemia, thrombocytopenia, coagulopathy, and blood product transfusions in critically ill patients.Clin Chest Med. 2003 Dec;24(4):607-22. doi: 10.1016/s0272-5231(03)00100-x. Clin Chest Med. 2003. PMID: 14710693 Review.
Cited by
-
Platelet function testing: Current practice among clinical centres in Northern Europe.Haemophilia. 2022 Jul;28(4):642-648. doi: 10.1111/hae.14578. Epub 2022 May 5. Haemophilia. 2022. PMID: 35510959 Free PMC article.
-
Exploring the relationship between blood platelet and other components utilizing count regression: A cross-sectional study in Bangladesh.Health Sci Rep. 2024 Aug 20;7(8):e70007. doi: 10.1002/hsr2.70007. eCollection 2024 Aug. Health Sci Rep. 2024. PMID: 39170887 Free PMC article.
-
Flow cytometry for evaluating platelet immunophenotyping and function in patients with thrombocytopenia.Tzu Chi Med J. 2022 Jul 26;34(4):381-387. doi: 10.4103/tcmj.tcmj_117_22. eCollection 2022 Oct-Dec. Tzu Chi Med J. 2022. PMID: 36578648 Free PMC article. Review.
-
Microfluidic System-Based Quantitative Analysis of Platelet Function through Speckle Size Measurement.Biomolecules. 2024 May 23;14(6):612. doi: 10.3390/biom14060612. Biomolecules. 2024. PMID: 38927016 Free PMC article.
-
Identification of High Platelet Reactivity Despite ADP P2Y 12 Inhibitor Treatment: Two Populations in the Vasodilator-Stimulated Phosphoprotein Assay and Variable PFA-P2Y Shapes of Curve.TH Open. 2023 Jun 7;7(2):e143-e154. doi: 10.1055/a-2075-7979. eCollection 2023 Apr. TH Open. 2023. PMID: 37292433 Free PMC article.
References
-
- Mazzano D., Pereira J. Approach to the patient with platelet-related bleeding. In: Gresele P., Kleiman N.S., Lopez J.A., Page C.P., editors. Platelets in Thrombotic and Non-Thrombotic Disorders: Pathophysiology, Pharmacology and Therapeutics: An Update. Springer International Publishing; Cham, Switzerland: 2017. pp. 717–725.
-
- Harrison P., Mackie I., Mumford A., Briggs C., Liesner R., Winter M., Machin S., British Committee for Standards in Haematology Guidelines for the laboratory investigation of heritable disorders of platelet function. Br. J. Haematol. 2011;155:30–44. doi: 10.1111/j.1365-2141.2011.08793.x. - DOI - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

