Antibacterial Activity of Positively and Negatively Charged Hematite (α-Fe2O3) Nanoparticles to Escherichia coli, Staphylococcus aureus and Vibrio fischeri
- PMID: 33800165
- PMCID: PMC7999532
- DOI: 10.3390/nano11030652
Antibacterial Activity of Positively and Negatively Charged Hematite (α-Fe2O3) Nanoparticles to Escherichia coli, Staphylococcus aureus and Vibrio fischeri
Abstract
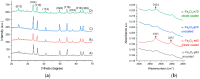
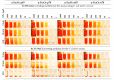
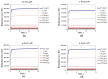
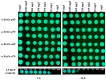
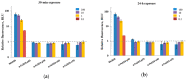
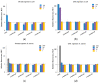
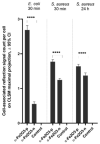
In the current study, the antibacterial activity of positively and negatively charged spherical hematite (α-Fe2O3) nanoparticles (NPs) with primary size of 45 and 70 nm was evaluated against clinically relevant bacteria Escherichia coli (gram-negative) and Staphylococcus aureus (gram-positive) as well as against naturally bioluminescent bacteria Vibrio fischeri (an ecotoxicological model organism). α-Fe2O3 NPs were synthesized using a simple green hydrothermal method and the surface charge was altered via citrate coating. To minimize the interference of testing environment with NP's physic-chemical properties, E. coli and S. aureus were exposed to NPs in deionized water for 30 min and 24 h, covering concentrations from 1 to 1000 mg/L. The growth inhibition was evaluated following the postexposure colony-forming ability of bacteria on toxicant-free agar plates. The positively charged α-Fe2O3 at concentrations from 100 mg/L upwards showed inhibitory activity towards E. coli already after 30 min of contact. Extending the exposure to 24 h caused total inhibition of growth at 100 mg/L. Bactericidal activity of positively charged hematite NPs against S. aureus was not observed up to 1000 mg/L. Differently from positively charged hematite NPs, negatively charged citrate-coated α-Fe2O3 NPs did not exhibit any antibacterial activity against E. coli and S. aureus even at 1000 mg/L. Confocal laser scanning microscopy and flow cytometer analysis showed that bacteria were more tightly associated with positively charged α-Fe2O3 NPs than with negatively charged citrate-coated α-Fe2O3 NPs. Moreover, the observed associations were more evident in the case of E. coli than S. aureus, being coherent with the toxicity results. Vibrio fischeri bioluminescence inhibition assays (exposure medium 2% NaCl) and colony forming ability on agar plates showed no (eco)toxicity of α-Fe2O3 (EC50 and MBC > 1000 mg/L).
Keywords: MicrobeJ; antibacterial; confocal; environmental safety; hematite; hydrothermal synthesis; nano-bio interactions; surface charge; α-Fe2O3 nanoparticles.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures






References
-
- Brushan M., Kumar Y., Periyasamy L., Viswanath A.K. Antibacterial applications of ɑ-Fe2O3/Co3O4 nanocomposites and study of their structural, optical, magnetic and cytotoxic characteristics. Appl. Nanosci. 2018;8:137–153. doi: 10.1007/s13204-018-0656-5. - DOI
-
- Abbaszadegan A., Ghahramani Y., Gholami A., Hemmateenejad B., Dorostkar S., Nabavizadeh M., Sharghi H. The effect of charge at the surface of silver nanoparticles on antimicrobial activity against gram-positive and gram-negative bacteria: A preliminary study. J. Nanomater. 2015;2015:720654. doi: 10.1155/2015/720654. - DOI
-
- Gabrielyan L., Hovhannisyn A., Gevorqyan V., Ananyan M., Trchounian A. Antibacterial effects of iron oxide (Fe3O4) nanoparticles: Distinguishing concentration-dependent effects with different bacterial cells growth and membrane-associated mechanisms. Appl. Microbiol. Biotechnol. 2019;103:2773–2782. doi: 10.1007/s00253-019-09653-x. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

