The Role of Tumor Necrosis Factor Alpha (TNF-α) in Autoimmune Disease and Current TNF-α Inhibitors in Therapeutics
- PMID: 33800290
- PMCID: PMC7962638
- DOI: 10.3390/ijms22052719
The Role of Tumor Necrosis Factor Alpha (TNF-α) in Autoimmune Disease and Current TNF-α Inhibitors in Therapeutics
Abstract
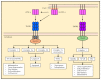
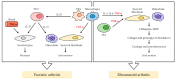
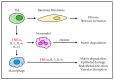
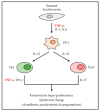
Tumor necrosis factor alpha (TNF-α) was initially recognized as a factor that causes the necrosis of tumors, but it has been recently identified to have additional important functions as a pathological component of autoimmune diseases. TNF-α binds to two different receptors, which initiate signal transduction pathways. These pathways lead to various cellular responses, including cell survival, differentiation, and proliferation. However, the inappropriate or excessive activation of TNF-α signaling is associated with chronic inflammation and can eventually lead to the development of pathological complications such as autoimmune diseases. Understanding of the TNF-α signaling mechanism has been expanded and applied for the treatment of immune diseases, which has resulted in the development of effective therapeutic tools, including TNF-α inhibitors. Currently, clinically approved TNF-α inhibitors have shown noticeable potency in a variety of autoimmune diseases, and novel TNF-α signaling inhibitors are being clinically evaluated. In this review, we briefly introduce the impact of TNF-α signaling on autoimmune diseases and its inhibitors, which are used as therapeutic agents against autoimmune diseases.
Keywords: TNF-α; TNF-α inhibitors; autoimmune diseases; inflammatory bowel disease; psoriatic arthritis; rheumatoid arthritis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
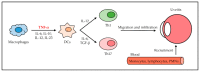
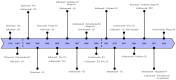
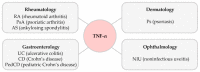
References
-
- Jiang Y., Yu M., Hu X., Han L., Yang K., Ba H., Zhang Z., Yin B., Yang X.P., Li Z., et al. STAT1 mediates transmembrane TNF-alpha-induced formation of death-inducing signaling complex and apoptotic signaling via TNFR1. Cell Death Differ. 2017;24:660–671. doi: 10.1038/cdd.2016.162. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

