The Emerging Role of Extracellular Vesicles in Endocrine Resistant Breast Cancer
- PMID: 33800302
- PMCID: PMC7962645
- DOI: 10.3390/cancers13051160
The Emerging Role of Extracellular Vesicles in Endocrine Resistant Breast Cancer
Abstract
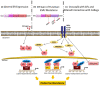
Breast cancer is the most common solid malignancy diagnosed in females worldwide, and approximately 70% of these tumors express estrogen receptor α (ERα), the main biomarker of endocrine therapy. Unfortunately, despite the use of long-term anti-hormone adjuvant treatment, which has significantly reduced patient mortality, resistance to the endocrine treatments often develops, leading to disease recurrence and limiting clinical benefits. Emerging evidence indicates that extracellular vesicles (EVs), nanosized particles that are released by all cell types and responsible for local and systemic intercellular communications, might represent a newly identified mechanism underlying endocrine resistance. Unraveling the role of EVs, released by transformed cells during the tumor evolution under endocrine therapy, is still an open question in the cancer research area and the molecular mechanisms involved should be better defined to discover alternative therapeutic approaches to overcome resistance. In this review, we will provide an overview of recent findings on the involvement of EVs in sustaining hormonal resistance in breast cancer and discuss opportunities for their potential use as biomarkers to monitor the therapeutic response and disease progression.
Keywords: breast cancer; endocrine resistance; exosomes; extracellular vesicles; targeted therapies.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Schiff R., Massarweh S.A., Shou J., Bharwani L., Arpino G., Rimawi M., Osborne C.K. Advanced concepts in estrogen receptor biology and breast cancer endocrine resistance: Implicated role of growth factor signaling and estrogen receptor coregulators. Cancer Chemother. Pharmacol. 2005;56:10–20. doi: 10.1007/s00280-005-0108-2. - DOI - PubMed
Publication types
Grants and funding
- Department of Excellence, Italian Law 232/2016/Ministero dell'Istruzione, dell'Università e della Ricerca
- Investigator Grant (IG) #21414/Associazione Italiana per la Ricerca sul Cancro
- BANDO PRIN 2017 # 2017EKMFTN_001/Ministero dell'Istruzione, dell'Università e della Ricerca
- BANDO PRIN 2017 # 2017WNKSLR_005/Ministero dell'Istruzione, dell'Università e della Ricerca
LinkOut - more resources
Full Text Sources
Other Literature Sources

