Oxidized Albumin as a Mediator of Kidney Disease
- PMID: 33800425
- PMCID: PMC8000637
- DOI: 10.3390/antiox10030404
Oxidized Albumin as a Mediator of Kidney Disease
Abstract
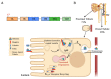
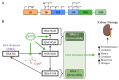
Renal diseases are a global health concern, and nearly 24% of kidney disease patients are overweight or obese. Particularly, increased body mass index has been correlated with oxidative stress and urinary albumin excretion in kidney disease patients, also contributing to increased cardiovascular risk. Albumin is the main plasma protein and is able to partially cross the glomerular filtration barrier, being reabsorbed mainly by the proximal tubule through different mechanisms. However, it has been demonstrated that albumin suffers different posttranslational modifications, including oxidation, which appears to be tightly linked to kidney damage progression and is increased in obese patients. Plasma-oxidized albumin levels correlate with a decrease in estimated glomerular filtration rate and an increase in blood urea nitrogen in patients with chronic kidney disease. Moreover, oxidized albumin in kidney disease patients is independently correlated with higher plasma levels of transforming growth factor beta (TGF-β1), tumor necrosis factor (TNF-α), and interleukin (IL)-1β and IL-6. In addition, oxidized albumin exerts a direct effect on neutrophils by augmenting the levels of neutrophil gelatinase-associated lipocalin, a well-accepted biomarker for renal damage in patients and in different experimental settings. Moreover, it has been suggested that albumin oxidation occurs at early stages of chronic kidney disease, accelerating the patient requirements for dialytic treatment during disease progression. In this review, we summarize the evidence supporting the role of overweight- and obesity-induced oxidative stress as a critical factor for the progression of renal disease and cardiovascular morbimortality through albumin oxidation.
Keywords: kidney disease; obesity; oxidized albumin.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
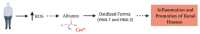
Similar articles
-
Proteinuria: detection and role in native renal disease progression.Transplant Rev (Orlando). 2012 Jan;26(1):3-13. doi: 10.1016/j.trre.2011.10.002. Transplant Rev (Orlando). 2012. PMID: 22137726 Review.
-
The changes of serum sKlotho and NGAL levels and their correlation in type 2 diabetes mellitus patients with different stages of urinary albumin.Diabetes Res Clin Pract. 2014 Nov;106(2):343-50. doi: 10.1016/j.diabres.2014.08.026. Epub 2014 Sep 16. Diabetes Res Clin Pract. 2014. PMID: 25263500
-
Albumin oxidation leads to neutrophil activation in vitro and inaccurate measurement of serum albumin in patients with diabetic nephropathy.Free Radic Biol Med. 2013 Jul;60:49-55. doi: 10.1016/j.freeradbiomed.2013.02.005. Epub 2013 Feb 18. Free Radic Biol Med. 2013. PMID: 23429046
-
Oxidized Albumin: Evaluation of Oxidative Stress as a Marker for the Progression of Kidney Disease.Biol Pharm Bull. 2022;45(12):1728-1732. doi: 10.1248/bpb.b22-00586. Biol Pharm Bull. 2022. PMID: 36450526
-
Subclinical Kidney Damage in Hypertensive Patients: A Renal Window Opened on the Cardiovascular System. Focus on Microalbuminuria.Adv Exp Med Biol. 2017;956:279-306. doi: 10.1007/5584_2016_85. Adv Exp Med Biol. 2017. PMID: 27873229 Review.
Cited by
-
Alterations in Autophagic Function and Endoplasmic Reticulum Stress Markers in the Peripheral Blood Mononuclear Cells of Patients on Hemodialysis.Int J Mol Sci. 2025 Jan 7;26(2):447. doi: 10.3390/ijms26020447. Int J Mol Sci. 2025. PMID: 39859163 Free PMC article.
-
Understanding acute kidney injury in cirrhosis: Current perspective.World J Hepatol. 2025 May 27;17(5):104724. doi: 10.4254/wjh.v17.i5.104724. World J Hepatol. 2025. PMID: 40501465 Free PMC article. Review.
-
Oxidized Albumin Induces Renal Tubular Cell Death and Promotes the Progression of Renal Diseases Through Ferroptosis.Int J Mol Sci. 2025 Jun 20;26(13):5924. doi: 10.3390/ijms26135924. Int J Mol Sci. 2025. PMID: 40649703 Free PMC article.
-
Lactate dehydrogenase-to-albumin ratio: A superior inflammatory marker for predicting contrast-associated acute kidney injury after percutaneous coronary intervention.Clin Cardiol. 2024 Feb;47(2):e24219. doi: 10.1002/clc.24219. Clin Cardiol. 2024. PMID: 38402549 Free PMC article.
-
Antioxidant Properties of Albumin and Diseases Related to Obstetrics and Gynecology.Antioxidants (Basel). 2025 Jan 6;14(1):55. doi: 10.3390/antiox14010055. Antioxidants (Basel). 2025. PMID: 39857389 Free PMC article. Review.
References
-
- World Health Organization. [(accessed on 11 February 2021)]; Available online: https://www.who.int/health-topics/obesity#tab=tab_3.
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

