Adaptive Changes in the Central Control of Energy Homeostasis Occur in Response to Variations in Energy Status
- PMID: 33800452
- PMCID: PMC7962960
- DOI: 10.3390/ijms22052728
Adaptive Changes in the Central Control of Energy Homeostasis Occur in Response to Variations in Energy Status
Abstract
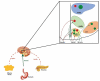
Energy homeostasis is regulated in coordinate fashion by the brain-gut axis, the homeostatic energy balance circuitry in the hypothalamus and the hedonic energy balance circuitry comprising the mesolimbcortical A10 dopamine pathway. Collectively, these systems convey and integrate information regarding nutrient status and the rewarding properties of ingested food, and formulate it into a behavioral response that attempts to balance fluctuations in consumption and food-seeking behavior. In this review we start with a functional overview of the homeostatic and hedonic energy balance circuitries; identifying the salient neural, hormonal and humoral components involved. We then delve into how the function of these circuits differs in males and females. Finally, we turn our attention to the ever-emerging roles of nociceptin/orphanin FQ (N/OFQ) and pituitary adenylate cyclase-activating polypeptide (PACAP)-two neuropeptides that have garnered increased recognition for their regulatory impact in energy homeostasis-to further probe how the imposed regulation of energy balance circuitry by these peptides is affected by sex and altered under positive (e.g., obesity) and negative (e.g., fasting) energy balance states. It is hoped that this work will impart a newfound appreciation for the intricate regulatory processes that govern energy homeostasis, as well as how recent insights into the N/OFQ and PACAP systems can be leveraged in the treatment of conditions ranging from obesity to anorexia.
Keywords: estradiol; fasting; nociceptin/orphanin FQ; obesity; pituitary adenylate cyclase-activating polypeptide; sex difference.
Conflict of interest statement
The authors declare no conflict of interest.
Figures






Similar articles
-
The PACAP Paradox: Dynamic and Surprisingly Pleiotropic Actions in the Central Regulation of Energy Homeostasis.Front Endocrinol (Lausanne). 2022 Jun 1;13:877647. doi: 10.3389/fendo.2022.877647. eCollection 2022. Front Endocrinol (Lausanne). 2022. PMID: 35721722 Free PMC article. Review.
-
Nociceptin/orphanin FQ modulates energy homeostasis through inhibition of neurotransmission at VMN SF-1/ARC POMC synapses in a sex- and diet-dependent manner.Biol Sex Differ. 2019 Feb 12;10(1):9. doi: 10.1186/s13293-019-0220-3. Biol Sex Differ. 2019. PMID: 30755252 Free PMC article.
-
PACAP in the Defense of Energy Homeostasis.Trends Endocrinol Metab. 2016 Sep;27(9):620-632. doi: 10.1016/j.tem.2016.04.008. Epub 2016 May 8. Trends Endocrinol Metab. 2016. PMID: 27166671 Review.
-
Contribution of thermogenic mechanisms by male and female mice lacking pituitary adenylate cyclase-activating polypeptide in response to cold acclimation.Am J Physiol Endocrinol Metab. 2021 Mar 1;320(3):E475-E487. doi: 10.1152/ajpendo.00205.2020. Epub 2020 Dec 28. Am J Physiol Endocrinol Metab. 2021. PMID: 33356993
-
Role of cAMP and K(+) channel-dependent mechanisms in piglet hypoxic/ischemic impaired nociceptin/orphanin FQ-induced cerebrovasodilation.Brain Res. 2000 Nov 24;884(1--2):51-8. doi: 10.1016/s0006-8993(00)02882-1. Brain Res. 2000. PMID: 11082486
Cited by
-
Female reproductive functions of the neuropeptide PACAP.Front Endocrinol (Lausanne). 2022 Sep 20;13:982551. doi: 10.3389/fendo.2022.982551. eCollection 2022. Front Endocrinol (Lausanne). 2022. PMID: 36204113 Free PMC article. Review.
-
The role of pituitary adenylate cyclase-activating polypeptide neurons in the hypothalamic ventromedial nucleus and the cognate PAC1 receptor in the regulation of hedonic feeding.Front Nutr. 2024 Aug 21;11:1437526. doi: 10.3389/fnut.2024.1437526. eCollection 2024. Front Nutr. 2024. PMID: 39234295 Free PMC article.
-
Pituitary Adenylate Cyclase Activating Polypeptide Inhibits A10 Dopamine Neurons and Suppresses the Binge-like Consumption of Palatable Food.Neuroscience. 2021 Dec 1;478:49-64. doi: 10.1016/j.neuroscience.2021.09.016. Epub 2021 Sep 28. Neuroscience. 2021. PMID: 34597709 Free PMC article.
-
The PACAP Paradox: Dynamic and Surprisingly Pleiotropic Actions in the Central Regulation of Energy Homeostasis.Front Endocrinol (Lausanne). 2022 Jun 1;13:877647. doi: 10.3389/fendo.2022.877647. eCollection 2022. Front Endocrinol (Lausanne). 2022. PMID: 35721722 Free PMC article. Review.
-
Molecular Mechanisms behind Obesity and Their Potential Exploitation in Current and Future Therapy.Int J Mol Sci. 2024 Jul 27;25(15):8202. doi: 10.3390/ijms25158202. Int J Mol Sci. 2024. PMID: 39125772 Free PMC article. Review.
References
-
- Hernandez J., Fabelo C., Perez L., Moore C., Chang R., Wagner E.J. Nociceptin/orphanin FQ modulates energy homeostasis through inhibition of neurotransmission at VMN SF-1/ARC POMC synapses in a sex- and diet-dependent manner. Biol. Sex Diff. 2019;10 doi: 10.1186/s13293-019-0220-3. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

