Unusually Fast bis-Histidyl Coordination in a Plant Hemoglobin
- PMID: 33800498
- PMCID: PMC7962945
- DOI: 10.3390/ijms22052740
Unusually Fast bis-Histidyl Coordination in a Plant Hemoglobin
Abstract
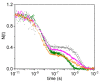
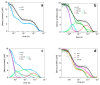
The recently identified nonsymbiotic hemoglobin gene MtGlb1-2 of the legume Medicago truncatula possesses unique properties as it generates four alternative splice forms encoding proteins with one or two heme domains. Here we investigate the ligand binding kinetics of MtGlb1-2.1 and MtGlb1-2.4, bearing two hemes and one heme, respectively. Unexpectedly, the overall time-course of ligand rebinding was unusually fast. Thus, we complemented nanosecond laser flash photolysis kinetics with data collected with a hybrid femtosecond-nanosecond pump-probe setup. Most photodissociated ligands are rebound geminately within a few nanoseconds, which leads to rates of the bimolecular rebinding to pentacoordinate species in the 108 M-1s-1 range. Binding of the distal histidine to the heme competes with CO rebinding with extremely high rates (kh ~ 105 s-1). Histidine dissociation from the heme occurs with comparable rates, thus resulting in moderate equilibrium binding constants (KH ~ 1). The rate constants for ligation and deligation of distal histidine to the heme are the highest reported for any plant or vertebrate globin. The combination of microscopic rates results in unusually high overall ligand binding rate constants, a fact that contributes to explaining at the mechanistic level the extremely high reactivity of these proteins toward the physiological ligands oxygen, nitric oxide and nitrite.
Keywords: CO rebinding kinetics; Medicago truncatula; iron/heme hexacoordination; plant hemoglobins; ultrafast spectroscopy.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



Similar articles
-
Bis-histidyl hexacoordination in hemoglobins facilitates heme reduction kinetics.J Am Chem Soc. 2004 Sep 29;126(38):11930-5. doi: 10.1021/ja046990w. J Am Chem Soc. 2004. PMID: 15382928
-
Measurement of distal histidine coordination equilibrium and kinetics in hexacoordinate hemoglobins.Methods Enzymol. 2008;436:359-78. doi: 10.1016/S0076-6879(08)36020-0. Methods Enzymol. 2008. PMID: 18237643
-
A Plant Gene Encoding One-Heme and Two-Heme Hemoglobins With Extreme Reactivities Toward Diatomic Gases and Nitrite.Front Plant Sci. 2020 Nov 19;11:600336. doi: 10.3389/fpls.2020.600336. eCollection 2020. Front Plant Sci. 2020. PMID: 33329665 Free PMC article.
-
Structure and reactivity of hexacoordinate hemoglobins.Biophys Chem. 2010 Nov;152(1-3):1-14. doi: 10.1016/j.bpc.2010.08.008. Epub 2010 Sep 21. Biophys Chem. 2010. PMID: 20933319 Free PMC article. Review.
-
Characterization of ligand migration mechanisms inside hemoglobins from the analysis of geminate rebinding kinetics.Methods Enzymol. 2008;437:329-45. doi: 10.1016/S0076-6879(07)37017-1. Methods Enzymol. 2008. PMID: 18433636 Review.
Cited by
-
Kinetic and dynamical properties of truncated hemoglobins of the Antarctic bacterium Pseudoalteromonas haloplanktis TAC125.Protein Sci. 2024 Jul;33(7):e5064. doi: 10.1002/pro.5064. Protein Sci. 2024. PMID: 38864722 Free PMC article.
References
-
- Appleby C.A. The origin and functions of hemoglobin in plants. Sci. Prog. 1992;76:365–398.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

