Activation of a Cryptic Manumycin-Type Biosynthetic Gene Cluster of Saccharothrix espanaensis DSM44229 by Series of Genetic Manipulations
- PMID: 33800500
- PMCID: PMC8000086
- DOI: 10.3390/microorganisms9030559
Activation of a Cryptic Manumycin-Type Biosynthetic Gene Cluster of Saccharothrix espanaensis DSM44229 by Series of Genetic Manipulations
Abstract
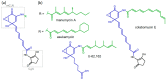
(1) Background: Manumycins are small actinomycete polyketides with prominent cancerostatic and immunosuppressive activities via inhibition of various eukaryotic enzymes. Their overall activity towards human cells depends on the structural variability of both their polyketide chains, mainly the upper one. In our genetic screening project to find novel producers of anti-inflammatory manumycins, the strain Saccharothrix espanaensis DSM44229 was identified as containing a novel manumycin-type biosynthetic gene cluster (BGC). (2) Methods: The biosynthetic genes appeared to be silent under all assayed laboratory conditions. Several techniques were used to activate the BGC, including: (i) heterologous expression in various hosts, (ii) overexpression of putative pathway-specific regulatory genes, and (iii) overexpression of a bottleneck cyclizing aminolevulinate synthase gene in both natural and heterologous producers. (3) Results: Multiple novel manumycin-type compounds were produced at various levels by genetically-modified strains, sharing a tetraene lower chain structure with a colabomycin subgroup of manumycins, but possessing much shorter and saturated upper chains. (4) Conclusions: A cryptic manumycin-type BGC was successfully activated by genetic means to gain production of novel manumycin-type compounds for future comparative activity assays. Heterologously produced compounds were identical to those found after final activation of the BGC in the original strain, proving the intactness of the cloned BGC.
Keywords: Saccharothrix; actinomycetes; cancerostatics; colabomycin; cryptic BGC activation; immunomodulators; manumycin; secondary metabolites.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures




Similar articles
-
Biosynthesis of colabomycin E, a new manumycin-family metabolite, involves an unusual chain-length factor.Chembiochem. 2014 Jun 16;15(9):1334-45. doi: 10.1002/cbic.201400068. Epub 2014 May 18. Chembiochem. 2014. PMID: 24838618
-
Inhibition of Pro-Inflammatory Cytokines by Metabolites of Streptomycetes-A Potential Alternative to Current Anti-Inflammatory Drugs?Microorganisms. 2020 Apr 25;8(5):621. doi: 10.3390/microorganisms8050621. Microorganisms. 2020. PMID: 32344935 Free PMC article.
-
Effect of starter unit availability on the spectrum of manumycin-type metabolites produced by Streptomyces nodosus ssp. asukaensis.J Appl Microbiol. 2011 Nov;111(5):1116-28. doi: 10.1111/j.1365-2672.2011.05132.x. Epub 2011 Sep 8. J Appl Microbiol. 2011. PMID: 21854515
-
Cloning and Heterologous Expression of a Large-sized Natural Product Biosynthetic Gene Cluster in Streptomyces Species.Front Microbiol. 2017 Mar 15;8:394. doi: 10.3389/fmicb.2017.00394. eCollection 2017. Front Microbiol. 2017. PMID: 28360891 Free PMC article. Review.
-
Activation of silent biosynthetic pathways and discovery of novel secondary metabolites in actinomycetes by co-culture with mycolic acid-containing bacteria.J Ind Microbiol Biotechnol. 2019 Mar;46(3-4):363-374. doi: 10.1007/s10295-018-2100-y. Epub 2018 Nov 28. J Ind Microbiol Biotechnol. 2019. PMID: 30488365 Review.
Cited by
-
Structure and Candidate Biosynthetic Gene Cluster of a Manumycin-Type Metabolite from Salinispora pacifica.J Nat Prod. 2022 Apr 22;85(4):980-986. doi: 10.1021/acs.jnatprod.1c01117. Epub 2022 Mar 9. J Nat Prod. 2022. PMID: 35263117 Free PMC article. Review.
-
Identification of Antimicrobial Compounds in Two Streptomyces sp. Strains Isolated From Beehives.Front Microbiol. 2022 Feb 3;13:742168. doi: 10.3389/fmicb.2022.742168. eCollection 2022. Front Microbiol. 2022. PMID: 35185841 Free PMC article.
-
Uncovering the genetic basis of antiviral polyketide limocrocin biosynthesis through heterologous expression.Microb Cell Fact. 2025 Jan 13;24(1):17. doi: 10.1186/s12934-024-02621-9. Microb Cell Fact. 2025. PMID: 39800732 Free PMC article.
References
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

