The Addition of Transdermal Delivery of Neostigmine and Glycopyrrolate by Iontophoresis to Thrice Weekly Bowel Care in Persons with Spinal Cord Injury: A Pilot Study
- PMID: 33800503
- PMCID: PMC7962943
- DOI: 10.3390/jcm10051135
The Addition of Transdermal Delivery of Neostigmine and Glycopyrrolate by Iontophoresis to Thrice Weekly Bowel Care in Persons with Spinal Cord Injury: A Pilot Study
Abstract
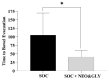
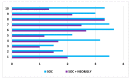
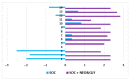
Persons with spinal cord injury (SCI) have neurogenic bowel disorders characterized by difficulty with evacuation (DWE), fecal incontinence, and discoordination of defecation. Six medically stable in-patients with SCI with a mean age of 57 ± 10 years (range: 39-66 years) and time since injury of 18 ± 17 years (range: 3-47 years) were investigated. Standard of care (SOC) for bowel care was followed by two weeks of SOC plus neostigmine (0.07 mg/kg) and glycopyrrolate (0.014 mg/kg) administered transcutaneously by iontophoresis thrice weekly for two weeks while patients continued to receive SOC. The primary endpoint was time to bowel evacuation. Body weights and abdominal radiographs were obtained. Ten questions related to bowel function and the Treatment Satisfaction Questionnaire for Medication were acquired after each arm. Bowel evacuation time decreased after the dual drug intervention arm (106.9 ± 68.4 vs. 40.8 ± 19.6 min; p < 0.0001). Body weight decreased (2.78 ± 0.98 kg; p < 0.0001), a finding confirmed on abdominal radiograph. Both questionnaires demonstrated improvement after the dual drug intervention arm. No major adverse events occurred. The addition of neostigmine and glycopyrrolate by transcutaneous administration to SOC for bowel care in persons with SCI and DWE resulted in the safe, effective, and predictable bowel evacuation with subjective improvement in bowel care.
Keywords: difficulty with evacuation; glycopyrrolate; iontophoresis; neostigmine; neurogenic bowel; spinal cord injury.
Conflict of interest statement
Authors (W.A.B. and M.A.K.) are the inventors of the dual drug combination of neostigmine and glycopyrrolate for the treatment of difficulty with evacuation. The Department of Veterans Affairs holds the patent to this invention.
Figures

References
-
- Pires J.M., Ferreira A.M., Rocha F., Andrade L.G., Campos I., Margalho P., Laíns J. Assessment of neurogenic bowel dysfunction impact after spinal cord injury using the International Classification of Functioning, Disability and Health. Eur. J. Phys. Rehabil. Med. 2019;54:873–879. doi: 10.23736/S1973-9087.18.04991-2. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

