Antioxidant Compound, Oxyresveratrol, Inhibits APP Production through the AMPK/ULK1/mTOR-Mediated Autophagy Pathway in Mouse Cortical Astrocytes
- PMID: 33800526
- PMCID: PMC7998742
- DOI: 10.3390/antiox10030408
Antioxidant Compound, Oxyresveratrol, Inhibits APP Production through the AMPK/ULK1/mTOR-Mediated Autophagy Pathway in Mouse Cortical Astrocytes
Abstract
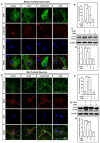
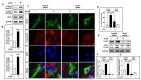
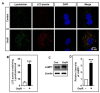
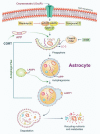
Oxyresveratrol (OxyR), a well-known polyphenolic phytoalexin, possesses a wide range of pharmacological and biological properties, comprising antioxidant, anti-inflammatory, free radical scavenging, anti-cancer, and neuroprotective activities. Autophagy is a cellular self-degradation system that removes aggregated or misfolded intracellular components via the autophagosome-lysosomal pathway. Astrocyte accumulation is one of the earliest neuropathological changes in Alzheimer's disease (AD), and amyloid precursor protein (APP) is the hallmark of AD. OxyR could affect APP modulation via the autophagy pathway. Here, we have reported that OxyR promotes autophagy signaling and attenuates APP production in primary cortical astrocytes based on immunofluorescence and immunoblotting assay results. Co-treatment with the late-stage autophagy inhibitor chloroquine (CQ) and OxyR caused significantly higher microtubule-associated protein light chain 3 (LC3)-II protein levels and LC3 puncta counts, demonstrating that OxyR stimulated autophagic flux. We also found that OxyR significantly reduced the levels of the autophagy substrate p62/SQSTM1, and p62 levels were significantly augmented by co-treatment with OxyR and CQ, because of the impaired deficiency of p62 in autolysosome. Likewise, pretreatment with the autophagy inhibitor, 3-methyladenine (3-MA), resulted in significantly fewer OxyR-induced LC3 puncta and lower LC3-II expression, suggesting that OxyR-mediated autophagy was dependent on the class III PI3-kinase pathway. In contrast, OxyR caused significantly lower LC3-II protein expression when pretreated with compound C, an AMP-activated protein kinase (AMPK) inhibitor, indicating that AMPK signaling regulated the OxyR-induced autophagic pathway. Additionally, co-treatment with OxyR with rapamycin intended to inhibit the mammalian target of rapamycin (mTOR) caused significantly lower levels of phospho-S6 ribosomal protein (pS6) and higher LC3-II expression, implying that OxyR-mediated autophagy was dependent on the mTOR pathway. Conversely, OxyR treatment significantly upregulated unc-51-like autophagy activating kinase 1 (ULK1) expression, and ULK1 small interfering RNAs (siRNA) caused significantly lower OxyR-induced LC3 puncta counts and LC3-II expression, indicating that ULK1 was essential for initiating OxyR-induced autophagy. However, we found that OxyR treatment astrocytes significantly increased the expression of lysosome-associated membrane protein 1 (LAMP1). Finally, we established a stress-induced APP production model using corticosterone (CORT) in cortical astrocytes, which produced significantly more APP than the equivalent using dexamethasone (DEX). In our experiment we found that CORT-induced APP production was significantly attenuated by OxyR through the autophagy pathway. Therefore, our study reveals that OxyR regulates AMPK/ULK1/mTOR-dependent autophagy induction and APP reduction in mouse cortical astrocytes.
Keywords: AMPK-ULK1; LC3 puncta; amyloid precursor protein (APP); autophagy; mTOR; oxyresveratrol.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



Similar articles
-
Platelet autophagic machinery involved in thrombosis through a novel linkage of AMPK-MTOR to sphingolipid metabolism.Autophagy. 2021 Dec;17(12):4141-4158. doi: 10.1080/15548627.2021.1904495. Epub 2021 Apr 5. Autophagy. 2021. PMID: 33749503 Free PMC article.
-
Magnolol improves Alzheimer's disease-like pathologies and cognitive decline by promoting autophagy through activation of the AMPK/mTOR/ULK1 pathway.Biomed Pharmacother. 2023 May;161:114473. doi: 10.1016/j.biopha.2023.114473. Epub 2023 Mar 6. Biomed Pharmacother. 2023. PMID: 36889111
-
Salidroside attenuates hypoxia-induced pulmonary arterial smooth muscle cell proliferation and apoptosis resistance by upregulating autophagy through the AMPK-mTOR-ULK1 pathway.BMC Pulm Med. 2017 Dec 12;17(1):191. doi: 10.1186/s12890-017-0477-4. BMC Pulm Med. 2017. PMID: 29233105 Free PMC article.
-
Impaired autophagy and APP processing in Alzheimer's disease: The potential role of Beclin 1 interactome.Prog Neurobiol. 2013 Jul-Aug;106-107:33-54. doi: 10.1016/j.pneurobio.2013.06.002. Epub 2013 Jul 1. Prog Neurobiol. 2013. PMID: 23827971 Review.
-
Targeting autophagy using small-molecule compounds to improve potential therapy of Parkinson's disease.Acta Pharm Sin B. 2021 Oct;11(10):3015-3034. doi: 10.1016/j.apsb.2021.02.016. Epub 2021 Feb 26. Acta Pharm Sin B. 2021. PMID: 34729301 Free PMC article. Review.
Cited by
-
Drug Target to Alleviate Mitochondrial Dysfunctions in Alzheimer's Disease: Recent Advances and Therapeutic Implications.Curr Neuropharmacol. 2024;22(12):1942-1959. doi: 10.2174/1570159X22666240426091311. Curr Neuropharmacol. 2024. PMID: 39234772 Free PMC article. Review.
-
Advancements in Utilizing Natural Compounds for Modulating Autophagy in Liver Cancer: Molecular Mechanisms and Therapeutic Targets.Cells. 2024 Jul 12;13(14):1186. doi: 10.3390/cells13141186. Cells. 2024. PMID: 39056768 Free PMC article. Review.
-
Targeting Neurological Disorders with Stilbenes: Bridging the Preclinical-Clinical Gap.Int J Biol Sci. 2024 Oct 7;20(14):5474-5494. doi: 10.7150/ijbs.102032. eCollection 2024. Int J Biol Sci. 2024. PMID: 39494329 Free PMC article. Review.
-
Recent Update and Drug Target in Molecular and Pharmacological Insights into Autophagy Modulation in Cancer Treatment and Future Progress.Cells. 2023 Jan 31;12(3):458. doi: 10.3390/cells12030458. Cells. 2023. PMID: 36766800 Free PMC article. Review.
-
Potential of Bioactive Food Components against Gastric Cancer: Insights into Molecular Mechanism and Therapeutic Targets.Cancers (Basel). 2021 Sep 7;13(18):4502. doi: 10.3390/cancers13184502. Cancers (Basel). 2021. PMID: 34572730 Free PMC article. Review.
References
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

