Early Administration of Convalescent Plasma Improves Survival in Patients with Hematological Malignancies and COVID-19
- PMID: 33800528
- PMCID: PMC8001057
- DOI: 10.3390/v13030436
Early Administration of Convalescent Plasma Improves Survival in Patients with Hematological Malignancies and COVID-19
Abstract
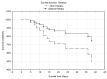
The use of convalescent plasma in the treatment of COVID-19 may lead to a milder course of infection and has been associated with improved outcomes. Determining optimal treatments in high risk populations is crucial, as is the case in those with hematological malignancies. We analyzed a cohort of 23 patients with hematological malignancies and COVID-19 who had received plasma 48-72 h after the diagnosis of infection and compared it with a historical group of 22 patients who received other therapy. Overall survival in those who received convalescent plasma was significantly higher than in the historical group (p = 0.03460). The plasma-treated group also showed a significantly milder course of infection (p = 0.03807), characterized by less severe symptoms and faster recovery (p = 0.00001). In conclusion, we have demonstrated that convalescent plasma is an effective treatment and its early administration leads to clinical improvement, increased viral clearance and longer overall survival in patients with hematological malignancies and COVID-19. To our knowledge, this is the first report to analyze the efficacy of convalescent plasma in a cohort of patients with hematological malignancies.
Keywords: COVID-19; convalescent plasma; hematological malignancies.
Conflict of interest statement
K.S. received consultancy honoraria, speaker fees, or research support from Roche, Gilead, Bayer, Abbvie, Intercept, Tobira, GSK, Janssen, MSD, BMS and Pfizer outside the submitted work. The remaining authors declare no competing financial interests. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures

References
-
- Biernat M.M., Zińczuk A., Biernat P., Bogucka-Fedorczuk A., Kwiatkowski J., Kalicińska E., Marciniak D., Simon K., Wróbel T. Nosocomial outbreak of SARS-CoV-2 infection in a haematological unit-High mortality rate in infected patients with haematologic malignancies. J. Clin. Virol. 2020;130:104574. doi: 10.1016/j.jcv.2020.104574. - DOI - PMC - PubMed
-
- Vijenthira A., Gong I.Y., Fox T.A., Booth S., Cook G., Fattizzo B., Martín-Moro F., Razanamahery J., Riches J.C., Zwicker J.I., et al. Outcomes of patients with hematologic malignancies and COVID-19: A systematic review and meta-analysis of 3377 patients. Blood. 2020;136:2881–2892. doi: 10.1182/blood.2020008824. - DOI - PMC - PubMed
-
- Lee L.Y.W., Cazier J.-B., Angelis V., Arnold R., Bisht V., Campton N.A., Chackathayil J., Cheng V.W., Curley H.M., Fittall M.W., et al. COVID-19 mortality in patients with cancer on chemotherapy or other anticancer treatments: A prospective cohort study. Lancet. 2020;395:1919–1926. doi: 10.1016/S0140-6736(20)31173-9. - DOI - PMC - PubMed
-
- Zarifkar P., Kamath A., Robinson C., Morgulchik N., Shah S., Cheng T., Dominic C., Fehintola A., Bhalla G., Ahillan T., et al. Clinical Characteristics and Outcomes in Patients with COVID-19 and Cancer: A Systematic Review and Meta-analysis. Clin. Oncol. 2021;33:e180–e191. doi: 10.1016/j.clon.2020.11.006. - DOI - PMC - PubMed
-
- Salazar E., Perez K.K., Ashraf M., Chen J., Castillo B., Christensen P.A., Eubank T., Bernard D.W., Eagar T.N., Long S.W., et al. Treatment of Coronavirus Disease 2019 (COVID-19) Patients with Convalescent Plasma. Am. J. Pathol. 2020;190:1680–1690. doi: 10.1016/j.ajpath.2020.05.014. - DOI - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

