Nef Obtained from Individuals with HIV-1 Vary in Their Ability to Antagonize SERINC3- and SERINC5-Mediated HIV-1 Restriction
- PMID: 33800773
- PMCID: PMC8000780
- DOI: 10.3390/v13030423
Nef Obtained from Individuals with HIV-1 Vary in Their Ability to Antagonize SERINC3- and SERINC5-Mediated HIV-1 Restriction
Abstract
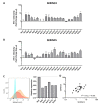
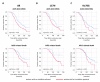
Nef is a multifunctional viral protein that has the ability to downregulate cell surface molecules, including CD4 and major histocompatibility complex class I (MHC-I) and, as recently shown, also members of the serine incorporator family (SERINC). Here, we analyzed the impact of naturally occurring mutations in HIV-1 Nef on its ability to counteract SERINC restriction and the clinical course of infection. HIV-1 Nef sequences were obtained from 123 participants of the Amsterdam Cohort Studies and showed multiple amino acid variations and mutations. Most of the primary Nef proteins showed increased activity to counteract SERINC3 and SERINC5 as compared to NL4-3 Nef. Several mutations in Nef were associated with either an increased or decreased infectivity of Bal26-pseudotyped HIV-1 produced in the presence of SERINC3 or SERINC5. The 8R, 157N and R178G Nef mutations were shown to have an effect on disease progression. Survival analysis showed an accelerated disease progression of individuals infected with HIV-1 carrying arginine or asparagine at position 8 or 157 in Nef, respectively, or the R178G Nef mutation. Here, we observed that naturally occurring mutations in Nef affect the ability of Nef to counteract SERINC3- and SERINC5-mediated inhibition of viral infectivity. The majority of these Nef mutations had no significant effect on HIV-1 pathogenesis and only the 8R, 157N and R178G mutations were associated with disease course.
Keywords: HIV-1; Nef; SERINC3; SERINC5; amino acid variations; disease progression; naturally occurring mutations.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures





References
-
- Alsahafi N., Ding S., Richard J., Markle T., Brassard N., Walker B., Lewis G.K., Kaufmann D.E., Brockman M.A., Finzi A. Nef proteins from HIV-1 elite controllers are inefficient at preventing antibody-dependent cellular cytotoxicity. J. Virol. 2015;90:2993–3002. doi: 10.1128/JVI.02973-15. - DOI - PMC - PubMed
-
- Le Gall S., Erdtmann L., Benichou S., Berlioz-Torrent C., Liu L., Benarous R., Heard J.M., Schwartz O. Nef interacts with the mu subunit of clathrin adaptor complexes and reveals a cryptic sorting signal in MHC I molecules. Immunity. 1998;8:483–495. doi: 10.1016/S1074-7613(00)80553-1. - DOI - PubMed
-
- Veillette M., Coutu M., Richard J., Batraville L.A., Dagher O., Bernard N., Tremblay C., Kaufmann D.E., Roger M., Finzi A. The HIV-1 GP120 CD4-bound conformation is preferentially targeted by antibody-dependent cellular cytotoxicity-mediating antibodies in sera from HIV-1-infected individuals. J. Virol. 2015;89:545–551. doi: 10.1128/JVI.02868-14. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

