Spectroscopic Signature of Red Blood Cells in a D-Galactose-Induced Accelerated Aging Model
- PMID: 33800818
- PMCID: PMC7961785
- DOI: 10.3390/ijms22052660
Spectroscopic Signature of Red Blood Cells in a D-Galactose-Induced Accelerated Aging Model
Abstract
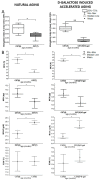
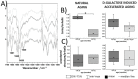
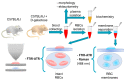
This work presents a semi-quantitative spectroscopic approach, including FTIR-ATR and Raman spectroscopies, for the biochemical analysis of red blood cells (RBCs) supported by the biochemical, morphological and rheological reference techniques. This multi-modal approach provided the description of the RBC alterations at the molecular level in a model of accelerated aging induced by administration of D-galactose (D-gal), in comparison to natural aging. Such an approach allowed to conclude that most age-related biochemical RBC membrane changes (a decrease in lipid unsaturation and the level of phospholipids, or an increase in acyl chain shortening) as well as alterations in the morphological parameters and RBC deformability are well reflected in the D-gal model of accelerated aging. Similarly, as in natural aging, a decrease in LDL level in blood plasma and no changes in the fraction of glucose, creatinine, total cholesterol, HDL, iron, or triglycerides were observed during the course of accelerated aging. Contrary to natural aging, the D-gal model led to an increase in cholesterol esters and the fraction of total esterified lipids in RBC membranes, and evoked significant changes in the secondary structure of the membrane proteins. Moreover, a significant decrease in the phosphorous level of blood plasma was specific for the D-gal model. On the other hand, natural aging induced stronger changes in the secondary structures of the proteins of the RBCs' interior. This work proves that research on the aging mechanism, especially in circulation-related diseases, should employ the D-gal model with caution. Nonetheless, the D-gal model enables to imitate age-related rheological alterations in RBCs, although they are partially derived from different changes observed in the RBC membrane at the molecular level.
Keywords: D-galactose-induced accelerated aging mouse model; Fourier transform infrared spectroscopy–attenuated total reflectance (FTIR–ATR); RBC membranes; Raman spectroscopy; aging; red blood cells (RBCs); vibrational spectroscopy.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures



References
-
- Flores M., Leonart M., Nascimento A. Oxidative process in erythrocytes of individuals with hemoglobin S. Hematology. 2008;13:187–192. - PubMed
-
- Dybas J., Bulat K., Blat A., Mohaissen T., Wajda A., Mardyla M., Kaczmarska M., Franczyk-Zarow M., Malek K., Chlopicki S., et al. Age-related and atherosclerosis-related erythropathy in ApoE/LDLR-/- mice. Biochim. Biophys. Acta Mol. Basis Dis. 2020:165972. doi: 10.1016/j.bbadis.2020.165972. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

