Tyrosine Kinase Inhibitors Are Promising Therapeutic Tools for Cats with HER2-Positive Mammary Carcinoma
- PMID: 33800900
- PMCID: PMC8002158
- DOI: 10.3390/pharmaceutics13030346
Tyrosine Kinase Inhibitors Are Promising Therapeutic Tools for Cats with HER2-Positive Mammary Carcinoma
Abstract
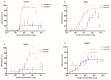
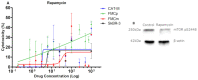
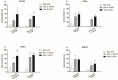
Feline mammary carcinoma (FMC) is a common neoplasia in cat, being HER2-positive the most prevalent subtype. In woman's breast cancer, tyrosine kinase inhibitors (TKi) are used as a therapeutic option, by blocking the phosphorylation of the HER2 tyrosine kinase domain. Moreover, clinical trials demonstrated that TKi produce synergistic antiproliferative effects in combination with mTOR inhibitors, overcoming resistance to therapy. Thus, to uncover new chemotherapeutic strategies for cats, the antiproliferative effects of two TKi (lapatinib and neratinib), and their combination with a mTOR inhibitor (rapamycin), were evaluated in FMC cell lines (CAT-M, FMCp and FMCm) and compared with a human breast cancer cell line (SkBR-3). Results revealed that both TKi induced antiproliferative effects in all feline cell lines, by blocking the phosphorylation of EGFR members and its downstream effectors. Furthermore, combined treatments with rapamycin presented synergetic antiproliferative effects. Additionally, the DNA sequence of the her2 TK domain (exons 18 to 20) was determined in 40 FMC tissue samples, and despite several mutations were found none of them were described as inducing resistance to therapy. Altogether, our results demonstrated that TKi and combined protocols may be useful in the treatment of cats with mammary carcinomas, and that TKi-resistant FMC are rare.
Keywords: HER2; feline her2 TK mutations; feline mammary carcinoma; targeted therapies; tyrosine kinase inhibitors.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures

Similar articles
-
HER2-Targeted Immunotherapy and Combined Protocols Showed Promising Antiproliferative Effects in Feline Mammary Carcinoma Cell-Based Models.Cancers (Basel). 2021 Apr 21;13(9):2007. doi: 10.3390/cancers13092007. Cancers (Basel). 2021. PMID: 33919468 Free PMC article.
-
Induction of HER2 Immunity in Outbred Domestic Cats by DNA Electrovaccination.Cancer Immunol Res. 2015 Jul;3(7):777-86. doi: 10.1158/2326-6066.CIR-14-0175. Epub 2015 Feb 23. Cancer Immunol Res. 2015. PMID: 25711535 Free PMC article.
-
Activation of mammalian target of rapamycin (mTOR) in triple negative feline mammary carcinomas.BMC Vet Res. 2013 Apr 15;9:80. doi: 10.1186/1746-6148-9-80. BMC Vet Res. 2013. PMID: 23587222 Free PMC article.
-
Preclinical Characteristics of the Irreversible Pan-HER Kinase Inhibitor Neratinib Compared with Lapatinib: Implications for the Treatment of HER2-Positive and HER2-Mutated Breast Cancer.Cancers (Basel). 2019 May 28;11(6):737. doi: 10.3390/cancers11060737. Cancers (Basel). 2019. PMID: 31141894 Free PMC article. Review.
-
Small molecule inhibitors targeting the EGFR/ErbB family of protein-tyrosine kinases in human cancers.Pharmacol Res. 2019 Jan;139:395-411. doi: 10.1016/j.phrs.2018.11.014. Epub 2018 Nov 27. Pharmacol Res. 2019. PMID: 30500458 Review.
Cited by
-
Comparative pharmacokinetics of tyrosine kinase inhibitor, lapatinib, in dogs and cats following single oral administration.J Vet Med Sci. 2024 Mar 16;86(3):317-321. doi: 10.1292/jvms.23-0448. Epub 2024 Jan 29. J Vet Med Sci. 2024. PMID: 38281758 Free PMC article.
-
Clinical Use of Molecular Biomarkers in Canine and Feline Oncology: Current and Future.Vet Sci. 2024 May 2;11(5):199. doi: 10.3390/vetsci11050199. Vet Sci. 2024. PMID: 38787171 Free PMC article. Review.
-
The Pivotal Role of Preclinical Animal Models in Anti-Cancer Drug Discovery and Personalized Cancer Therapy Strategies.Pharmaceuticals (Basel). 2024 Aug 9;17(8):1048. doi: 10.3390/ph17081048. Pharmaceuticals (Basel). 2024. PMID: 39204153 Free PMC article. Review.
-
A Scoping Review on Tyrosine Kinase Inhibitors in Cats: Current Evidence and Future Directions.Animals (Basel). 2023 Sep 29;13(19):3059. doi: 10.3390/ani13193059. Animals (Basel). 2023. PMID: 37835664 Free PMC article.
-
Emerging Biomarkers and Targeted Therapies in Feline Mammary Carcinoma.Vet Sci. 2021 Aug 11;8(8):164. doi: 10.3390/vetsci8080164. Vet Sci. 2021. PMID: 34437486 Free PMC article. Review.
References
-
- Porrello A., Cardelli P., Spugnini E.P. Oncology of companion animals as a model for humans. An overview of tumor histotypes. J. Exp. Clin. Cancer Res. 2006;25:97–105. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

