Dinaciclib, a Bimodal Agent Effective against Endometrial Cancer
- PMID: 33800911
- PMCID: PMC7962054
- DOI: 10.3390/cancers13051135
Dinaciclib, a Bimodal Agent Effective against Endometrial Cancer
Abstract
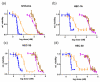
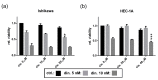
Endometrial cancer (EC) is the sixth most prevalent female cancer globally and although high rates of success are achieved when diagnosed at an early stage, the 5-year survival rate for cancers diagnosed at Stages II-IV is below 50%. Improving patient outcomes will necessitate the introduction of novel therapies to the clinic. Pan-cyclin-dependent kinase inhibitors (CDKis) have been explored as therapies for a range of cancers due to their ability to simultaneously target multiple key cellular processes, such as cell cycle progression, transcription, and DNA repair. Few studies, however, have reported on their potential for the treatment of EC. Herein, we examined the effects of the pan-CDKi dinaciclib in primary cells isolated directly from tumors and EC cell lines. Dinaciclib was shown to elicit a bimodal action in EC cell lines, disrupting both cell cycle progression and phosphorylation of the RNA polymerase carboxy terminal domain, with a concomitant reduction in Bcl-2 expression. Furthermore, the therapeutic potential of combining dinaciclib and cisplatin was explored, with the drugs demonstrating synergy at specific doses in Type I and Type II EC cell lines. Together, these results highlight the potential of dinaciclib for use as an effective EC therapy.
Keywords: CDK inhibitor; dinaciclib; endometrial cancer.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




Similar articles
-
Dinaciclib as an effective pan-cyclin dependent kinase inhibitor in platinum resistant ovarian cancer.Front Oncol. 2022 Nov 25;12:1014280. doi: 10.3389/fonc.2022.1014280. eCollection 2022. Front Oncol. 2022. PMID: 36505806 Free PMC article.
-
Pre-therapeutic efficacy of the CDK inhibitor dinaciclib in medulloblastoma cells.Sci Rep. 2021 Mar 8;11(1):5374. doi: 10.1038/s41598-021-84082-3. Sci Rep. 2021. PMID: 33686114 Free PMC article.
-
The multi-CDK inhibitor dinaciclib reverses bromo- and extra-terminal domain (BET) inhibitor resistance in acute myeloid leukemia via inhibition of Wnt/β-catenin signaling.Exp Hematol Oncol. 2024 Mar 4;13(1):27. doi: 10.1186/s40164-024-00483-w. Exp Hematol Oncol. 2024. PMID: 38438856 Free PMC article.
-
Dinaciclib for the treatment of breast cancer.Expert Opin Investig Drugs. 2014 Sep;23(9):1305-12. doi: 10.1517/13543784.2014.948152. Epub 2014 Aug 8. Expert Opin Investig Drugs. 2014. PMID: 25107301 Review.
-
Cyclin-dependent protein kinase inhibitors including palbociclib as anticancer drugs.Pharmacol Res. 2016 May;107:249-275. doi: 10.1016/j.phrs.2016.03.012. Epub 2016 Mar 16. Pharmacol Res. 2016. PMID: 26995305 Review.
Cited by
-
CDK9 inhibition as an effective therapy for small cell lung cancer.Cell Death Dis. 2024 May 20;15(5):345. doi: 10.1038/s41419-024-06724-4. Cell Death Dis. 2024. PMID: 38769311 Free PMC article.
-
Immune infiltration and drug specificity analysis of different subtypes based on functional status in angioimmunoblastic T-cell lymphoma.Heliyon. 2023 Aug 1;9(8):e18836. doi: 10.1016/j.heliyon.2023.e18836. eCollection 2023 Aug. Heliyon. 2023. PMID: 37576233 Free PMC article.
-
Rational drug combination design in patient-derived avatars reveals effective inhibition of hepatocellular carcinoma with proteasome and CDK inhibitors.J Exp Clin Cancer Res. 2022 Aug 15;41(1):249. doi: 10.1186/s13046-022-02436-9. J Exp Clin Cancer Res. 2022. PMID: 35971164 Free PMC article.
-
Cancer therapy by cyclin-dependent kinase inhibitors (CDKIs): bench to bedside.EXCLI J. 2024 Jun 4;23:862-882. doi: 10.17179/excli2024-7076. eCollection 2024. EXCLI J. 2024. PMID: 38983782 Free PMC article. Review.
-
In silico directed evolution of Anabas testudineus AtMP1 antimicrobial peptide to improve in vitro anticancer activity.PeerJ. 2024 Sep 26;12:e17894. doi: 10.7717/peerj.17894. eCollection 2024. PeerJ. 2024. PMID: 39346049 Free PMC article.
References
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

