Excess TGF-β1 Drives Cardiac Mesenchymal Stromal Cells to a Pro-Fibrotic Commitment in Arrhythmogenic Cardiomyopathy
- PMID: 33800912
- PMCID: PMC7961797
- DOI: 10.3390/ijms22052673
Excess TGF-β1 Drives Cardiac Mesenchymal Stromal Cells to a Pro-Fibrotic Commitment in Arrhythmogenic Cardiomyopathy
Abstract
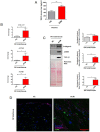
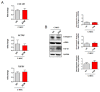
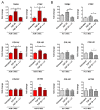
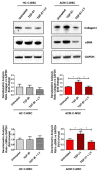
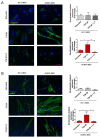
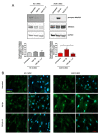
Arrhythmogenic Cardiomyopathy (ACM) is characterized by the replacement of the myocardium with fibrotic or fibro-fatty tissue and inflammatory infiltrates in the heart. To date, while ACM adipogenesis is a well-investigated differentiation program, ACM-related fibrosis remains a scientific gap of knowledge. In this study, we analyze the fibrotic process occurring during ACM pathogenesis focusing on the role of cardiac mesenchymal stromal cells (C-MSC) as a source of myofibroblasts. We performed the ex vivo studies on plasma and right ventricular endomyocardial bioptic samples collected from ACM patients and healthy control donors (HC). In vitro studies were performed on C-MSC isolated from endomyocardial biopsies of both groups. Our results revealed that circulating TGF-β1 levels are significantly higher in the ACM cohort than in HC. Accordingly, fibrotic markers are increased in ACM patient-derived cardiac biopsies compared to HC ones. This difference is not evident in isolated C-MSC. Nevertheless, ACM C-MSC are more responsive than HC ones to TGF-β1 treatment, in terms of pro-fibrotic differentiation and higher activation of the SMAD2/3 signaling pathway. These results provide the novel evidence that C-MSC are a source of myofibroblasts and participate in ACM fibrotic remodeling, being highly responsive to ACM-characteristic excess TGF-β1.
Keywords: TGF-β1; arrhythmogenic cardiomyopathy; cardiac remodeling; cardiac-mesenchymal stromal cells; fibrosis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Casella M., Gasperetti A., Sicuso R., Conte E., Catto V., Sommariva E., Bergonti M., Vettor G., Rizzo S., Pompilio G., et al. Characteristics of Patients With Arrhythmogenic Left Ventricular Cardiomyopathy: Combining Genetic and Histopathologic Findings. Circ. Arrhythm. Electrophysiol. 2020;13:e009005. doi: 10.1161/CIRCEP.120.009005. - DOI - PubMed
-
- Lin C.Y., Lin Y.J., Li C.H., Chung F.P., Lo M.T., Lin C., Chang H.C., Chang S.L., Lo L.W., Hu Y.F., et al. Heterogeneous distribution of substrates between the endocardium and epicardium promotes ventricular fibrillation in arrhythmogenic right ventricular dysplasia/cardiomyopathy. Eurospace. 2018;20:501–511. doi: 10.1093/europace/euw393. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

