Baker's Yeast-Based Microbial Fuel Cell Mediated by 2-Methyl-1,4-Naphthoquinone
- PMID: 33800926
- PMCID: PMC8001437
- DOI: 10.3390/membranes11030182
Baker's Yeast-Based Microbial Fuel Cell Mediated by 2-Methyl-1,4-Naphthoquinone
Abstract
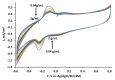
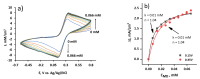
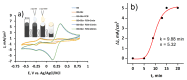
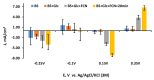
Microbial fuel cell (MFC) efficiency depends on charge transfer capability from microbe to anode, and the application of suitable redox mediators is important in this area. In this study, yeast viability experiments were performed to determine the 2-methyl-1,4-naphthoquinone (menadione (MD)) influence on different yeast cell species (baker's yeast and Saccharomyces cerevisiae yeast cells). In addition, electrochemical measurements to investigate MFC performance and efficiency were carried out. This research revealed that baker's yeast cells were more resistant to dissolved MD, but the current density decreased when yeast solution concentration was incrementally increased in the same cell. The maximal calculated power of a designed baker's yeast-based MFC cell anode was 0.408 mW/m2 and this power output was registered at 24 mV. Simultaneously, the cell generated a 62-mV open circuit potential in the presence of 23 mM potassium ferricyanide and the absence of glucose and immobilized MD. The results only confirm that MD has strong potential to be applied to microbial fuel cells and that a two-redox-mediator-based system is suitable for application in microbial fuel cells.
Keywords: 2-methyl-1,4-naphthoquinone; Saccharomyces cerevisiae; baker’s yeast cells; biofuel cell; graphite rod electrode; microbial fuel cell.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Rahimnejad M., Adhami A., Darvari S., Zirepour A., Oh S.E. Microbial fuel cell as new technology for bioelectricity generation: A review. Alex. Eng. J. 2015;54:745–756. doi: 10.1016/j.aej.2015.03.031. - DOI
-
- Rahimnejad M., Ghoreyshi A.A., Najafpour G., Jafary T. Power generation from organic substrate in batch and continuous flow microbial fuel cell operations. Appl. Energy. 2011 doi: 10.1016/j.apenergy.2011.04.017. - DOI
-
- Slate A.J., Whitehead K.A., Brownson D.A.C., Banks C.E. Microbial fuel cells: An overview of current technology. Renew. Sustain. Energy Rev. 2019;101:60–81. doi: 10.1016/j.rser.2018.09.044. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

