Saccharomyces cerevisiae Concentrates Subtoxic Copper onto Cell Wall from Solid Media Containing Reducing Sugars as Carbon Source
- PMID: 33800957
- PMCID: PMC8000517
- DOI: 10.3390/bioengineering8030036
Saccharomyces cerevisiae Concentrates Subtoxic Copper onto Cell Wall from Solid Media Containing Reducing Sugars as Carbon Source
Abstract
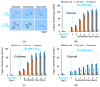
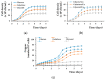
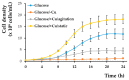
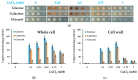
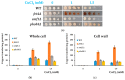
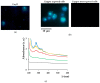
Copper is essential for life, but it can be deleterious in concentrations that surpass the physiological limits. Copper pollution is related to widespread human activities, such as viticulture and wine production. To unravel aspects of how organisms cope with copper insults, we used Saccharomyces cerevisiae as a model for adaptation to high but subtoxic concentrations of copper. We found that S. cerevisiae cells could tolerate high copper concentration by forming deposits on the cell wall and that the copper-containing deposits accumulated predominantly when cells were grown statically on media prepared with reducing sugars (glucose, galactose) as sole carbon source, but not on media containing nonreducing carbon sources, such as glycerol or lactate. Exposing cells to copper in liquid media under strong agitation prevented the formation of copper-containing deposits at the cell wall. Disruption of low-affinity copper intake through the plasma membrane increased the potential of the cell to form copper deposits on the cell surface. These results imply that biotechnology problems caused by high copper concentration can be tackled by selecting yeast strains and conditions to allow the removal of excess copper from various contaminated sites in the forms of solid deposits which do not penetrate the cell.
Keywords: Saccharomyces cerevisiae; carbon source; coloration; copper; extracellular deposit.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Accumulation of Ag(I) by Saccharomyces cerevisiae Cells Expressing Plant Metallothioneins.Cells. 2018 Dec 11;7(12):266. doi: 10.3390/cells7120266. Cells. 2018. PMID: 30545005 Free PMC article.
-
Depression of Saccharomyces cerevisiae invasive growth on non-glucose carbon sources requires the Snf1 kinase.Mol Microbiol. 2002 Jul;45(2):453-69. doi: 10.1046/j.1365-2958.2002.03024.x. Mol Microbiol. 2002. PMID: 12123456
-
[The age-dependent effect of copper ions on different strains of the wine yeast (Saccharomyces [corrected] cerevisiae var vini)].Georgian Med News. 2008 Sep;(162):34-9. Georgian Med News. 2008. PMID: 18830028 Russian.
-
The activity of plasma membrane H(+)-ATPase is strongly stimulated during Saccharomyces cerevisiae adaptation to growth under high copper stress, accompanying intracellular acidification.Yeast. 2001 Apr;18(6):511-21. doi: 10.1002/yea.702. Yeast. 2001. PMID: 11284007
-
Glycerol as a substrate for Saccharomyces cerevisiae based bioprocesses - Knowledge gaps regarding the central carbon catabolism of this 'non-fermentable' carbon source.Biotechnol Adv. 2019 Nov 1;37(6):107378. doi: 10.1016/j.biotechadv.2019.03.017. Epub 2019 Mar 28. Biotechnol Adv. 2019. PMID: 30930107 Review.
Cited by
-
Engineering Biology of Yeast for Advanced Biomanufacturing.Bioengineering (Basel). 2022 Dec 21;10(1):10. doi: 10.3390/bioengineering10010010. Bioengineering (Basel). 2022. PMID: 36671581 Free PMC article.
-
Characterisation of putative class 1A DHODH-like proteins from Mucorales and dematiaceous mould species.PLoS One. 2023 Aug 2;18(8):e0289441. doi: 10.1371/journal.pone.0289441. eCollection 2023. PLoS One. 2023. PMID: 37531380 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

