Leflunomide Sustained Skin Delivery Based on Sulfobetaine-Modified Chitosan Nanoparticles Embedded in Biodegradable Polyesters Films
- PMID: 33800966
- PMCID: PMC8003864
- DOI: 10.3390/polym13060960
Leflunomide Sustained Skin Delivery Based on Sulfobetaine-Modified Chitosan Nanoparticles Embedded in Biodegradable Polyesters Films
Abstract
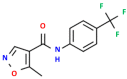
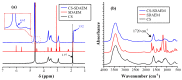
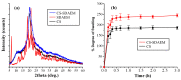
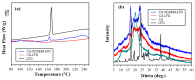
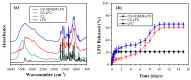
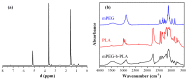
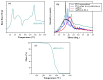
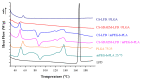
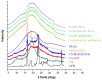
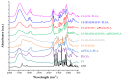
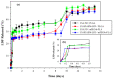
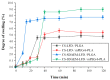
The aim of the present study was to prepare a leflunomide (LFD) sustained release transdermal delivery system for the treatment of psoriasis. In this context, LFD-loaded nanoparticles (NPs) based on either neat chitosan (CS) or CS modified with [2-(methacryloyloxy)ethyl]dimethyl-(3-sulfopropyl)ammonium hydroxide (SDAEM, a sulfobetaine zwitterionic compound) were initially prepared via ionotropic gelation and characterized in terms of in vitro dissolution, physicochemical, and antibacterial properties. Results showed that the use of the SDAEM-modified CS resulted in the formation of LFD-loaded NPs with improved wetting and solubilization properties, better in vitro dissolution profile characteristics (i.e., higher dissolution rate and extent), and improved (enhanced) antibacterial properties. The resultant LFD-loaded NPs were then embedded in suitable thin-film skin patches, prepared via spin-coating, utilizing two different biodegradable polyesters, namely methoxy polyethylene glycol-b-poly(L-lactide) (mPEG-b-PLA, at a ratio of 25/75 mPEG to PLA) and poly(lactic-co-glycolic acid) (PLGA at a ratio of 75/25 DL-lactide/glycolide copolymer). Results showed the formation of polymeric thin-films with no agglomeration (or trapped air) and uniform structure in all cases, while the LFD-loaded NPs were successfully embedded in the polymeric matrix. Analysis of the obtained in vitro dissolution profiles revealed a sustained release profile of the drug for up to approximately twelve days, while between the two proposed systems, the use of CS-SDAEM NPs (independently of the polyester type) was the most promising formulation approach.
Keywords: PLGA; SDAEM-grafting; biodegradable polyesters; chitosan; ionotropic gelation; leflunomide; mPEG-b-PLA; nanoparticles; spin coating; thin-film patches.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
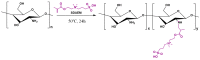
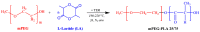

Similar articles
-
Leflunomide Loaded Chitosan Nanoparticles for the Preparation of Aliphatic Polyester Based Skin Patches.Polymers (Basel). 2021 May 11;13(10):1539. doi: 10.3390/polym13101539. Polymers (Basel). 2021. PMID: 34064952 Free PMC article.
-
Poly(lactide)-vitamin E derivative/montmorillonite nanoparticle formulations for the oral delivery of Docetaxel.Biomaterials. 2009 Jul;30(19):3297-306. doi: 10.1016/j.biomaterials.2009.02.045. Epub 2009 Mar 19. Biomaterials. 2009. PMID: 19299012
-
Effect of polyethylene glycol (PEG) chain organization on the physicochemical properties of poly(D, L-lactide) (PLA) based nanoparticles.Eur J Pharm Biopharm. 2010 Jun;75(2):96-106. doi: 10.1016/j.ejpb.2010.03.002. Epub 2010 Mar 6. Eur J Pharm Biopharm. 2010. PMID: 20211727
-
Lipid-polymer hybrid nanoparticles as a new generation therapeutic delivery platform: a review.Eur J Pharm Biopharm. 2013 Nov;85(3 Pt A):427-43. doi: 10.1016/j.ejpb.2013.07.002. Epub 2013 Jul 17. Eur J Pharm Biopharm. 2013. PMID: 23872180 Review.
-
Pegylated poly(lactide) and poly(lactide-co-glycolide) nanoparticles: preparation, properties and possible applications in drug delivery.Curr Drug Deliv. 2004 Oct;1(4):321-33. doi: 10.2174/1567201043334605. Curr Drug Deliv. 2004. PMID: 16305394 Review.
Cited by
-
Fabrication of PLA-Based Nanoneedle Patches Loaded with Transcutol-Modified Chitosan Nanoparticles for the Transdermal Delivery of Levofloxacin.Molecules. 2024 Sep 10;29(18):4289. doi: 10.3390/molecules29184289. Molecules. 2024. PMID: 39339284 Free PMC article.
-
Chitosan-Based Hydrogels for Controlled Delivery of Asiaticoside-Rich Centella asiatica Extracts with Wound Healing Potential.Int J Mol Sci. 2023 Dec 7;24(24):17229. doi: 10.3390/ijms242417229. Int J Mol Sci. 2023. PMID: 38139059 Free PMC article.
-
L-Cysteine Modified Chitosan Nanoparticles and Carbon-Based Nanostructures for the Intranasal Delivery of Galantamine.Polymers (Basel). 2022 Sep 24;14(19):4004. doi: 10.3390/polym14194004. Polymers (Basel). 2022. PMID: 36235952 Free PMC article.
References
-
- Sala M., Elaissari A., Fessi H. Advances in psoriasis physiopathology and treatments: Up to date of mechanistic insights and perspectives of novel therapies based on innovative skin drug delivery systems (ISDDS) J. Control Release. 2016;239:182–202. doi: 10.1016/j.jconrel.2016.07.003. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

