Implication of Dietary Iron-Chelating Bioactive Compounds in Molecular Mechanisms of Oxidative Stress-Induced Cell Ageing
- PMID: 33800975
- PMCID: PMC8003849
- DOI: 10.3390/antiox10030491
Implication of Dietary Iron-Chelating Bioactive Compounds in Molecular Mechanisms of Oxidative Stress-Induced Cell Ageing
Abstract
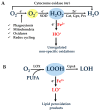
One of the prevailing perceptions regarding the ageing of cells and organisms is the intracellular gradual accumulation of oxidatively damaged macromolecules, leading to the decline of cell and organ function (free radical theory of ageing). This chemically undefined material known as "lipofuscin," "ceroid," or "age pigment" is mainly formed through unregulated and nonspecific oxidative modifications of cellular macromolecules that are induced by highly reactive free radicals. A necessary precondition for reactive free radical generation and lipofuscin formation is the intracellular availability of ferrous iron (Fe2+) ("labile iron"), catalyzing the conversion of weak oxidants such as peroxides, to extremely reactive ones like hydroxyl (HO•) or alcoxyl (RO•) radicals. If the oxidized materials remain unrepaired for extended periods of time, they can be further oxidized to generate ultimate over-oxidized products that are unable to be repaired, degraded, or exocytosed by the relevant cellular systems. Additionally, over-oxidized materials might inactivate cellular protection and repair mechanisms, thus allowing for futile cycles of increasingly rapid lipofuscin accumulation. In this review paper, we present evidence that the modulation of the labile iron pool distribution by nutritional or pharmacological means represents a hitherto unappreciated target for hampering lipofuscin accumulation and cellular ageing.
Keywords: Mediterranean diet; ageing mechanisms; bioactive dietary compounds; cellular senescence; free radicals; iron-chelating agents; labile iron; oxidative stress.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Oxidative Stress-Induced Cellular Senescence: Is Labile Iron the Connecting Link?Antioxidants (Basel). 2023 Jun 10;12(6):1250. doi: 10.3390/antiox12061250. Antioxidants (Basel). 2023. PMID: 37371980 Free PMC article. Review.
-
Considerations on the mechanism of action of artemisinin antimalarials: part 1--the 'carbon radical' and 'heme' hypotheses.Infect Disord Drug Targets. 2013 Aug;13(4):217-77. doi: 10.2174/1871526513666131129155708. Infect Disord Drug Targets. 2013. PMID: 24304352 Review.
-
Combined administration of membrane-permeable and impermeable iron-chelating drugs attenuates ischemia/reperfusion-induced hepatic injury.Free Radic Biol Med. 2022 Nov 20;193(Pt 1):227-237. doi: 10.1016/j.freeradbiomed.2022.10.266. Epub 2022 Oct 13. Free Radic Biol Med. 2022. PMID: 36243210
-
The labile iron pool attenuates peroxynitrite-dependent damage and can no longer be considered solely a pro-oxidative cellular iron source.J Biol Chem. 2018 Jun 1;293(22):8530-8542. doi: 10.1074/jbc.RA117.000883. Epub 2018 Apr 16. J Biol Chem. 2018. PMID: 29661935 Free PMC article.
-
Lipofuscin and ceroid formation: the cellular recycling system.Adv Exp Med Biol. 1989;266:3-15. doi: 10.1007/978-1-4899-5339-1_1. Adv Exp Med Biol. 1989. PMID: 2486157 Review.
Cited by
-
Functional MOF-Based Materials for Environmental and Biomedical Applications: A Critical Review.Nanomaterials (Basel). 2023 Jul 31;13(15):2224. doi: 10.3390/nano13152224. Nanomaterials (Basel). 2023. PMID: 37570542 Free PMC article. Review.
-
Oxidative Stress-Induced Cellular Senescence: Is Labile Iron the Connecting Link?Antioxidants (Basel). 2023 Jun 10;12(6):1250. doi: 10.3390/antiox12061250. Antioxidants (Basel). 2023. PMID: 37371980 Free PMC article. Review.
-
Exploring the Role of Metabolic Hyperferritinaemia (MHF) in Steatotic Liver Disease (SLD) and Hepatocellular Carcinoma (HCC).Cancers (Basel). 2025 Feb 28;17(5):842. doi: 10.3390/cancers17050842. Cancers (Basel). 2025. PMID: 40075688 Free PMC article. Review.
-
Impact of Piriformospora indica on various characteristics of tomatoes during nickel nitrate stress under aeroponic and greenhouse conditions.Front Microbiol. 2023 Feb 3;13:1091036. doi: 10.3389/fmicb.2022.1091036. eCollection 2022. Front Microbiol. 2023. PMID: 36817111 Free PMC article.
-
Dietary Antioxidants in the Mediterranean Diet.Antioxidants (Basel). 2021 Jul 28;10(8):1213. doi: 10.3390/antiox10081213. Antioxidants (Basel). 2021. PMID: 34439460 Free PMC article.
References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

