Nanostructures in Hydrogen Peroxide Sensing
- PMID: 33801140
- PMCID: PMC8004286
- DOI: 10.3390/s21062204
Nanostructures in Hydrogen Peroxide Sensing
Abstract
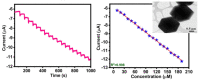
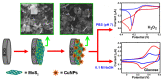
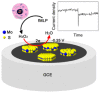
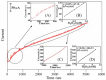
In recent years, several devices have been developed for the direct measurement of hydrogen peroxide (H2O2), a key compound in biological processes and an important chemical reagent in industrial applications. Classical enzymatic biosensors for H2O2 have been recently outclassed by electrochemical sensors that take advantage of material properties in the nano range. Electrodes with metal nanoparticles (NPs) such as Pt, Au, Pd and Ag have been widely used, often in combination with organic and inorganic molecules to improve the sensing capabilities. In this review, we present an overview of nanomaterials, molecules, polymers, and transduction methods used in the optimization of electrochemical sensors for H2O2 sensing. The different devices are compared on the basis of the sensitivity values, the limit of detection (LOD) and the linear range of application reported in the literature. The review aims to provide an overview of the advantages associated with different nanostructures to assess which one best suits a target application.
Keywords: biosensors; enzymes; hydrogen peroxide; nanostructures; sensors.
Conflict of interest statement
The authors declare no conflict of interest.
Figures











References
-
- Hurdis E.C., Romeyn H. Accuracy of Determination of Hydrogen Peroxide by Cerate Oxidimetry. Anal. Chem. 1954;26:320–325. doi: 10.1021/ac60086a016. - DOI
-
- Matsubara C., Kawamoto N., Takamura K. Oxo[5, 10, 15, 20-tetra(4-pyridyl)porphyrinato]titanium(IV): An ultra-high sensitivity spectrophotometric reagent for hydrogen peroxide. Analyst. 1992;117:1781. doi: 10.1039/an9921701781. - DOI
-
- Nakashima K., Wada M., Kuroda N., Akiyama S., Imai K. High-Performance Liquid Chromatographic Determination of Hydrogen Peroxide with Peroxyoxalate Chemiluminescence Detection. J. Liq. Chromatogr. 1994;17:2111–2126. doi: 10.1080/10826079408013535. - DOI
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

