Clinical Effectiveness and Safety of Once-Weekly GLP-1 Receptor Agonist Dulaglutide as Add-On to Metformin or Metformin Plus Insulin Secretagogues in Obesity and Type 2 Diabetes
- PMID: 33801192
- PMCID: PMC7957905
- DOI: 10.3390/jcm10050985
Clinical Effectiveness and Safety of Once-Weekly GLP-1 Receptor Agonist Dulaglutide as Add-On to Metformin or Metformin Plus Insulin Secretagogues in Obesity and Type 2 Diabetes
Abstract
Aims and methods: The aim of this monocentric retrospective observational study was to evaluate the 18-month safety and effectiveness of GLP-1 receptor agonist (GLP-1 RA) dulaglutide (DU) 1.5 mg/once weekly as an add-on to metformin (MET) or MET plus conventional insulin secretagogues in a study cohort with excess body weight and type 2 diabetes (T2D). Comparative efficacy versus liraglutide (LIRA) 1.2-1.8 mg/once daily in a study sample naïve to GLP-1 RAs, frequency matching for age, gender, T2D duration, degree of glycemic impairment, cardiovascular comorbidities, and medications, was addressed as a secondary aim. Clinical and biochemical data for efficacy outcomes and information on drug discontinuation due to adverse events (AEs) were collected from digital records.
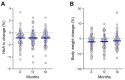
Results: Initial analysis included 126 overweight and obese T2D patients (48.4% females). Out of these, 13 discontinued DU due to moderate-severe gastrointestinal AEs after a mean follow-up of 6 (4 standard deviations (SD)) months, while 65 completed 18 months of continuous therapy. At 6 months, there was a significant mean HbA1c reduction of -0.85% (1.17 SD) with respect to baseline values (p < 0.001), which remained stable during 18 months follow-up. These results were accompanied by a moderate weight loss sustained over time, with a mean reduction of -2.0% (4.3 SD) at 6 months and -1.3% (4.8 SD) at 18 months (p = 0.091). At univariate analysis, a negative correlation between baseline body mass index (BMI) and risk of drug discontinuation due to gastrointestinal AEs was observed. The protective effect of obesity against drug discontinuation was confirmed by logistic regression analysis. Neither gender, nor age, nor T2D duration, nor concomitant conventional insulin secretagogue use, nor switching to DU from other GLP-1 RAs influenced its long-term effectiveness. However, higher baseline HbA1c values emerged as predictors of clinically relevant efficacy outcomes, either in terms of HbA1c reduction ≥ 0.5% or body weight loss ≥ 5%. The efficacy outcomes were corroborated by head-to-head comparison with LIRA, a GLP-1 RA with durable beneficial effects on glycemic control and body weight in real-world experiences. With the advantage of once-weekly administration, at 18-month follow-up, a significantly larger fraction of patients on DU therapy reached glycemic targets (HbA1c ≤ 7.0%) when compared to those on LIRA: from 14.8% at baseline (both groups) to 64.8% with DU and 42.6% with LIRA (p = 0.033).
Conclusions: Although limited by a retrospective design and lack of constant up-titration for LIRA to the highest dose, these findings indicate that the beneficial responses to DU on a background of MET or MET plus insulin secretagogues are durable, especially in the presence of obesity and greater HbA1c impairment.
Keywords: GLP-1 receptor agonist (GLP-1 RA); add-on therapy; dulaglutide; liraglutide; obesity; type 2 diabetes.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Madsen K.S., Kähler P., Kähler L.K., Madsbad S., Gnesin F., Metzendorf M.I., Richter B., Hemmingsen B. Metformin and second- or third-generation sulphonylurea combination therapy for adults with type 2 diabetes mellitus. Cochrane Database Syst. Rev. 2019;4:CD012368. doi: 10.1002/14651858.CD012368.pub2. - DOI - PMC - PubMed
-
- Giorda C.B., Orsi E., De Cosmo S., Bossi A.C., Guerzoni C., Cercone S., Gilio B., Cavalot F. Prescription of Sulphonylureas among Patients with Type 2 Diabetes Mellitus in Italy: Results from the Retrospective, Observational Multicentre Cross-Sectional SUSCIPE (Sulphonyl_UreaS_Correct_Internal_Prescription_Evaluation) Study. Diabetes Ther. 2020;11:2105–2119. doi: 10.1007/s13300-020-00871-5. - DOI - PMC - PubMed
-
- Potts J.E., Gray L.J., Brady E.M., Khunti K., Davies M.J., Bodicoat D.H. The effect of glucagon-like peptide 1 receptor agonists on weight Loss in type 2 diabetes: A systematic review and mixed treatment comparison meta-analysis. PLoS ONE. 2015;10:e0126769. doi: 10.1371/journal.pone.0126769. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

