Retinal Microvascular Impairment in COVID-19 Bilateral Pneumonia Assessed by Optical Coherence Tomography Angiography
- PMID: 33801324
- PMCID: PMC7998142
- DOI: 10.3390/biomedicines9030247
Retinal Microvascular Impairment in COVID-19 Bilateral Pneumonia Assessed by Optical Coherence Tomography Angiography
Abstract
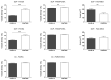
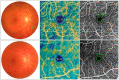
The purpose of this study was to evaluate the presence of retinal and microvascular alterations in COVID-19 patients with bilateral pneumonia due to SARS-COV-2 that required hospital admission and compare this with a cohort of age- and sex-matched controls. COVID-19 bilateral pneumonia patients underwent retinal imaging 14 days after hospital discharge with structural optical coherence tomography (OCT) and optical coherence tomography angiography (OCTA) measurements. Vessel density (VD) and foveal avascular zone (FAZ) area were evaluated in the superficial, deep capillary plexus (SCP, DCP), and choriocapillaris (CC). After exclusion criteria, only one eye per patient was selected, and 50 eyes (25 patients and 25 controls) were included in the analysis. COVID-19 patients presented significantly thinner ganglion cell layer (GCL) (p = 0.003) and thicker retinal nerve fiber layer (RNFL) compared to controls (p = 0.048), and this RNFL thickening was greater in COVID-19 cases with cotton wool spots (CWS), when compared with patients without CWS (p = 0.032). In both SCP and DCP, COVID-19 patients presented lower VD in the foveal region (p < 0.001) and a greater FAZ area than controls (p = 0.007). These findings suggest that thrombotic and inflammatory phenomena could be happening in the retina of COVID-19 patients. Further research is warranted to analyze the longitudinal evolution of these changes over time as well as their correlation with disease severity.
Keywords: COVID-19; OCT; OCTA; SARS-COV-2; coronavirus; cotton wool spot; microvascular; optical coherence tomography; optical coherence tomography angiography; retina.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
-
- Hoffmann M., Kleine-Weber H., Schroeder S., Krüger N., Herrler T., Erichsen S., Schiergens T.S., Herrler G., Wu N.H., Nitsche A., et al. SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor. Cell. 2020;181:271–280. doi: 10.1016/j.cell.2020.02.052. - DOI - PMC - PubMed
-
- Recalde-Zamacona B., García-Tobar L., Argueta A., Álvarez L., De Andrea C.E., Fernández Alonso M., Ezponda A., Carmona Torre F., Jordán Iborra C., Quiroga J.A., et al. Histopathological findings in fatal COVID-19 severe acute respiratory syndrome: Preliminary experience from a series of 10 Spanish patients. Thorax. 2020 doi: 10.1136/thoraxjnl-2020-215577. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

