Aflatoxin B1 and Aflatoxin M1 Induce Compromised Intestinal Integrity through Clathrin-Mediated Endocytosis
- PMID: 33801329
- PMCID: PMC8002210
- DOI: 10.3390/toxins13030184
Aflatoxin B1 and Aflatoxin M1 Induce Compromised Intestinal Integrity through Clathrin-Mediated Endocytosis
Abstract
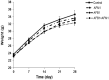
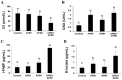
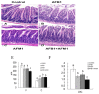
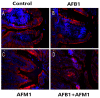
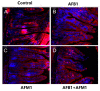
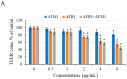
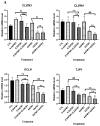
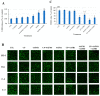
With the growing diversity and complexity of diet, humans are at risk of simultaneous exposure to aflatoxin B1 (AFB1) and aflatoxin M1 (AFM1), which are well-known contaminants in dairy and other agricultural products worldwide. The intestine represents the first barrier against external contaminants; however, evidence about the combined effect of AFB1 and AFM1 on intestinal integrity is lacking. In vivo, the serum biochemical parameters related to intestinal barrier function, ratio of villus height/crypt depth, and distribution pattern of claudin-1 and zonula occluden-1 were significantly affected in mice exposed to 0.3 mg/kg b.w. AFB1 and 3.0 mg/kg b.w. AFM1. In vitro results on differentiated Caco-2 cells showed that individual and combined AFB1 (0.5 and 4 μg/mL) and AFM1 (0.5 and 4 μg/mL) decreased cell viability and trans-epithelial electrical resistance values as well as increased paracellular permeability of fluorescein isothiocyanate-dextran in a dose-dependent manner. Furthermore, AFM1 aggravated AFB1-induced compromised intestinal barrier, as demonstrated by the down-regulation of tight junction proteins and their redistribution, particularly internalization. Adding the inhibitor chlorpromazine illustrated that clathrin-mediated endocytosis partially contributed to the compromised intestinal integrity. Synergistic and additive effects were the predominant interactions, suggesting that these toxins are likely to have negative effects on human health.
Keywords: aflatoxin B1; aflatoxin M1; endocytosis; intestinal epithelial barrier.
Conflict of interest statement
No potential competing interest was reported by the authors.
Figures



Similar articles
-
Omics analysis revealed the intestinal toxicity induced by aflatoxin B1 and aflatoxin M1.Ecotoxicol Environ Saf. 2024 Jun 15;278:116336. doi: 10.1016/j.ecoenv.2024.116336. Epub 2024 Apr 30. Ecotoxicol Environ Saf. 2024. PMID: 38691883
-
Modulation of Intestinal Epithelial Permeability in Differentiated Caco-2 Cells Exposed to Aflatoxin M1 and Ochratoxin A Individually or Collectively.Toxins (Basel). 2017 Dec 27;10(1):13. doi: 10.3390/toxins10010013. Toxins (Basel). 2017. PMID: 29280945 Free PMC article.
-
Aflatoxin B1 and aflatoxin M1 induced cytotoxicity and DNA damage in differentiated and undifferentiated Caco-2 cells.Food Chem Toxicol. 2015 Sep;83:54-60. doi: 10.1016/j.fct.2015.05.020. Epub 2015 Jun 5. Food Chem Toxicol. 2015. PMID: 26051350
-
Aflatoxin B1 and M1: Biological Properties and Their Involvement in Cancer Development.Toxins (Basel). 2018 May 24;10(6):214. doi: 10.3390/toxins10060214. Toxins (Basel). 2018. PMID: 29794965 Free PMC article. Review.
-
Aflatoxin M1 in milk and dairy products: The state of the evidence for child growth impairment.Food Chem Toxicol. 2024 Nov;193:115008. doi: 10.1016/j.fct.2024.115008. Epub 2024 Sep 18. Food Chem Toxicol. 2024. PMID: 39304086 Review.
Cited by
-
Aflatoxin B1: Challenges and Strategies for the Intestinal Microbiota and Intestinal Health of Monogastric Animals.Toxins (Basel). 2025 Jan 17;17(1):43. doi: 10.3390/toxins17010043. Toxins (Basel). 2025. PMID: 39852996 Free PMC article. Review.
-
Intestinal Barrier, Claudins and Mycotoxins.Toxins (Basel). 2021 Oct 26;13(11):758. doi: 10.3390/toxins13110758. Toxins (Basel). 2021. PMID: 34822542 Free PMC article. Review.
-
Mycotoxins of Concern in Children and Infant Cereal Food at European Level: Incidence and Bioaccessibility.Toxins (Basel). 2022 Jul 15;14(7):488. doi: 10.3390/toxins14070488. Toxins (Basel). 2022. PMID: 35878226 Free PMC article. Review.
-
The Protective Effects of Lactoferrin on Aflatoxin M1-Induced Compromised Intestinal Integrity.Int J Mol Sci. 2021 Dec 28;23(1):289. doi: 10.3390/ijms23010289. Int J Mol Sci. 2021. PMID: 35008712 Free PMC article.
-
Integrated Metabolomics and Lipidomics Analysis Reveals Lipid Metabolic Disorder in NCM460 Cells Caused by Aflatoxin B1 and Aflatoxin M1 Alone and in Combination.Toxins (Basel). 2023 Mar 31;15(4):255. doi: 10.3390/toxins15040255. Toxins (Basel). 2023. PMID: 37104193 Free PMC article.
References
-
- Medina Á., Rodríguez A., Magan N. Climate change and mycotoxigenic fungi: Impacts on mycotoxin production. Curr. Opin. Food Sci. 2015;5:99–104. doi: 10.1016/j.cofs.2015.11.002. - DOI
Publication types
MeSH terms
Substances
Grants and funding
- 31972190/National Natural Science Foundation of China
- 2004DA125184G1608/the Independent Research Project of State Key Laboratory of Animal Nutrition
- CARS-36/Modern Agro-Industry Technology Research System of the PR China
- CAAS-ZDXT2019004/the Scientific Research Project for Major Achievements of Agricultural Science and Technology Innovation Program
LinkOut - more resources
Full Text Sources
Other Literature Sources

