Mercury Chloride but Not Lead Acetate Causes Apoptotic Cell Death in Human Lung Fibroblast MRC5 Cells via Regulation of Cell Cycle Progression
- PMID: 33801350
- PMCID: PMC7958599
- DOI: 10.3390/ijms22052494
Mercury Chloride but Not Lead Acetate Causes Apoptotic Cell Death in Human Lung Fibroblast MRC5 Cells via Regulation of Cell Cycle Progression
Abstract
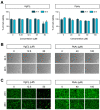
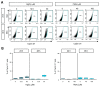
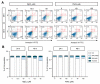
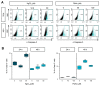
Heavy metals are important for various biological systems, but, in excess, they pose a serious risk to human health. Heavy metals are commonly used in consumer and industrial products. Despite the increasing evidence on the adverse effects of heavy metals, the detailed mechanisms underlying their action on lung cancer progression are still poorly understood. In the present study, we investigated whether heavy metals (mercury chloride and lead acetate) affect cell viability, cell cycle, and apoptotic cell death in human lung fibroblast MRC5 cells. The results showed that mercury chloride arrested the sub-G1 and G2/M phases by inducing cyclin B1 expression. In addition, the exposure to mercury chloride increased apoptosis through the activation of caspase-3. However, lead had no cytotoxic effects on human lung fibroblast MRC5 cells at low concentration. These findings demonstrated that mercury chloride affects the cytotoxicity of MRC5 cells by increasing cell cycle progression and apoptotic cell death.
Keywords: HgCl2; MRC5; PbAc; apoptosis; cell cycle arrest; heavy metal; human lung fibroblast cell.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



Similar articles
-
Transcriptome Analysis Reveals HgCl2 Induces Apoptotic Cell Death in Human Lung Carcinoma H1299 Cells through Caspase-3-Independent Pathway.Int J Mol Sci. 2021 Feb 18;22(4):2006. doi: 10.3390/ijms22042006. Int J Mol Sci. 2021. PMID: 33670495 Free PMC article.
-
Mitigative role of garlic and vitamin E against cytotoxic, genotoxic, and apoptotic effects of lead acetate and mercury chloride on WI-38 cells.Pharmacol Rep. 2018 Aug;70(4):804-811. doi: 10.1016/j.pharep.2018.02.009. Epub 2018 Feb 5. Pharmacol Rep. 2018. PMID: 29966875
-
[In vitro study of the nephrotoxic mechanism of mercuric chloride].Med Lav. 2002 May-Jun;93(3):267-78. Med Lav. 2002. PMID: 12197277 Italian.
-
Hg(II) exposure exacerbates UV-induced DNA damage in MRC5 fibroblasts: a comet assay study.J Environ Sci Health A Tox Hazard Subst Environ Eng. 2006;41(2):143-8. doi: 10.1080/10934520500349243. J Environ Sci Health A Tox Hazard Subst Environ Eng. 2006. PMID: 16423720
-
Cell death effects of resin-based dental material compounds and mercurials in human gingival fibroblasts.Arch Toxicol. 2006 Jun;80(6):370-7. doi: 10.1007/s00204-005-0044-2. Epub 2005 Nov 17. Arch Toxicol. 2006. PMID: 16691427
Cited by
-
Benzo(a)pyrene triggers cytotoxicity by disrupting cell cycle dynamics and activating Caspase-3-mediated apoptosis in multiple human cell lines.Toxicol Res (Camb). 2025 Apr 14;14(2):tfaf053. doi: 10.1093/toxres/tfaf053. eCollection 2025 Apr. Toxicol Res (Camb). 2025. PMID: 40236271
-
Inhalation Bioaccessibility and Risk Assessment of Metals in PM2.5 Based on a Multiple-Path Particle Dosimetry Model in the Smelting District of Northeast China.Int J Environ Res Public Health. 2022 Jul 22;19(15):8915. doi: 10.3390/ijerph19158915. Int J Environ Res Public Health. 2022. PMID: 35897292 Free PMC article.
-
TRIM27 revealing by tumor educated platelet RNA-sequencing, as a potential biomarker for malignant ground-glass opacities diagnosis mediates glycolysis of non-small cell lung cancer cells partially through HOXM1.Transl Lung Cancer Res. 2024 Sep 30;13(9):2307-2325. doi: 10.21037/tlcr-24-157. Epub 2024 Sep 24. Transl Lung Cancer Res. 2024. PMID: 39430321 Free PMC article.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

