Effects of Miosis on Anterior Chamber Structure in Glaucoma Implant Surgery
- PMID: 33801436
- PMCID: PMC7958613
- DOI: 10.3390/jcm10051017
Effects of Miosis on Anterior Chamber Structure in Glaucoma Implant Surgery
Abstract
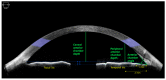
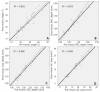
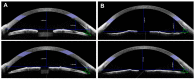
We investigated changes in anterior chamber (AC) structure after miosis in phakic eyes and pseudophakic eyes with glaucoma. In this prospective study, patients scheduled for glaucoma implant surgery were examined using anterior segment optical coherence tomography before and after miosis. Four AC parameters (AC angle, peripheral anterior chamber (PAC) depth, central anterior chamber (CAC) depth, and AC area) were analyzed before and after miosis, and then compared between phakic and pseudophakic eyes. Twenty-nine phakic eyes and 36 pseudophakic eyes were enrolled. The AC angle widened after miosis in both the phakia and pseudophakia groups (p = 0.019 and p < 0.001, respectively). In the phakia group, CAC depth (p < 0.001) and AC area (p = 0.02) were significantly reduced after miosis, and the reductions in PAC depth, CAC depth, and AC area were significantly greater than in the pseudophakia group (all p < 0.05). Twenty-five patients (86.2%) in the phakia group and 17 (47.2%) in the pseudophakia group had reduced CAC depth (p = 0.004). Although miosis increased the AC angle in both groups, AC depth decreased in most phakic eyes and a substantial number of pseudophakic eyes. Preoperative miosis before glaucoma implant surgery may interfere with implant tube placement distant from the cornea during insertion into the AC.
Keywords: anterior chamber; glaucoma implant surgery; miosis; pilocarpine.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Watson P., Barnett F. Effectiveness of trabeculectomy in glaucoma. Am. J. Ophthalmol. 1975;79:831–845. - PubMed
-
- Shaarawy T. Glaucoma, Volume 2: Surgical Management. Saunders Ltd.; Wynnewood, PA, USA: 2009.
-
- Kim J., Lee J., Kee C. Tissue incarceration after Ahmed valve implantation. J. Korean Ophthalmol. Soc. 2012;53:1053–1056.
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

