Prostacyclin Analogues Inhibit Platelet Reactivity, Extracellular Vesicle Release and Thrombus Formation in Patients with Pulmonary Arterial Hypertension
- PMID: 33801460
- PMCID: PMC7958838
- DOI: 10.3390/jcm10051024
Prostacyclin Analogues Inhibit Platelet Reactivity, Extracellular Vesicle Release and Thrombus Formation in Patients with Pulmonary Arterial Hypertension
Abstract
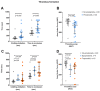
(1) Background: Prostacyclin analogues (epoprostenol, treprostinil, and iloprost) induce vasodilation in pulmonary arterial hypertension (PAH) but also inhibit platelet function. (2) Objectives: We assessed platelet function in PAH patients treated with prostacyclin analogues and not receiving prostacyclin analogues. (3) Methods: Venous blood was collected from 42 patients treated with prostacyclin analogues (49.5 ± 15.9 years, 81% female) and 38 patients not receiving prostacyclin analogues (55.5 ± 15.6 years, 74% female). Platelet reactivity was analyzed by impedance aggregometry using arachidonic acid (AA; 0.5 mM), adenosine diphosphate (ADP; 6.5 µM), and thrombin receptor-activating peptide (TRAP; 32 µM) as agonists. In a subset of patients, concentrations of extracellular vesicles (EVs) from all platelets (CD61+), activated platelets (CD61+/CD62P+), leukocytes (CD45+), and endothelial cells (CD146+) were analyzed by flow cytometry. Platelet-rich thrombus formation was measured using a whole blood perfusion system. (4) Results: Compared to controls, PAH patients treated with prostacyclin analogues had lower platelet reactivity in response to AA and ADP (p = 0.01 for both), lower concentrations of platelet and leukocyte EVs (p ≤ 0.04), delayed thrombus formation (p ≤ 0.003), and decreased thrombus size (p = 0.008). Epoprostenol did not affect platelet reactivity but decreased the concentrations of platelet and leukocyte EVs (p ≤ 0.04). Treprostinil decreased platelet reactivity in response to AA and ADP (p ≤ 0.02) but had no effect on the concentrations of EVs. All prostacyclin analogues delayed thrombus formation and decreased thrombus size (p ≤ 0.04). (5) Conclusions: PAH patients treated with prostacyclin analogues had impaired platelet reactivity, EV release, and thrombus formation, compared to patients not receiving prostacyclin analogues.
Keywords: extracellular vesicles; platelet reactivity; prostacyclin analogues; pulmonary arterial hypertension; thrombus formation.
Conflict of interest statement
E.v.d.P. is a cofounder and shareholder of Exometry B.V., M.K. received speakers fee and travel grants from Actelion, Bayer and AOP Orphan. All other authors report no declarations of interest.
Figures




Similar articles
-
Prostacyclin analogues decrease platelet aggregation but have no effect on thrombin generation, fibrin clot structure, and fibrinolysis in pulmonary arterial hypertension: PAPAYA coagulation.Platelets. 2022 Oct 3;33(7):1065-1074. doi: 10.1080/09537104.2022.2042234. Epub 2022 Mar 14. Platelets. 2022. PMID: 35285383
-
Effects of antiplatelet therapy on platelet extracellular vesicle release and procoagulant activity in health and in cardiovascular disease.Platelets. 2016 Dec;27(8):805-811. doi: 10.1080/09537104.2016.1190008. Epub 2016 Jun 16. Platelets. 2016. PMID: 27310292
-
Treprostinil treatment decreases circulating platelet microvesicles and their procoagulant activity in pediatric pulmonary hypertension.Pediatr Pulmonol. 2019 Jan;54(1):66-72. doi: 10.1002/ppul.24190. Epub 2018 Nov 28. Pediatr Pulmonol. 2019. PMID: 30485728
-
The prostacyclin pathway in pulmonary arterial hypertension: a clinical review.Expert Rev Respir Med. 2017 Jun;11(6):491-503. doi: 10.1080/17476348.2017.1317599. Epub 2017 Apr 24. Expert Rev Respir Med. 2017. PMID: 28399721 Review.
-
Prostacyclin for pulmonary arterial hypertension.Cochrane Database Syst Rev. 2019 May 1;5(5):CD012785. doi: 10.1002/14651858.CD012785.pub2. Cochrane Database Syst Rev. 2019. PMID: 31042010 Free PMC article.
Cited by
-
Higher EpCAM-Positive Extracellular Vesicle Concentration in Ascites Is Associated with Shorter Progression-Free Survival of Patients with Advanced High-Grade Serous Carcinoma.Int J Mol Sci. 2024 Jun 20;25(12):6780. doi: 10.3390/ijms25126780. Int J Mol Sci. 2024. PMID: 38928484 Free PMC article.
-
Multifunctional nanoparticle-mediated combining therapy for human diseases.Signal Transduct Target Ther. 2024 Jan 1;9(1):1. doi: 10.1038/s41392-023-01668-1. Signal Transduct Target Ther. 2024. PMID: 38161204 Free PMC article. Review.
-
Dynamics of oxylipin biosynthesis in systemic inflammation: insights from a large animal model of endotoxemia.Front Immunol. 2025 Jun 16;16:1595888. doi: 10.3389/fimmu.2025.1595888. eCollection 2025. Front Immunol. 2025. PMID: 40589758 Free PMC article.
-
Platelet function changes in patients undergoing endovascular aortic aneurysm repair: Review of the literature.Front Cardiovasc Med. 2022 Aug 12;9:927995. doi: 10.3389/fcvm.2022.927995. eCollection 2022. Front Cardiovasc Med. 2022. PMID: 36035918 Free PMC article. Review.
-
Compassionate use of Pulmonary Vasodilators in Acute Severe Hypoxic Respiratory Failure due to COVID-19.J Intensive Care Med. 2022 Aug;37(8):1101-1111. doi: 10.1177/08850666221086521. Epub 2022 Apr 4. J Intensive Care Med. 2022. PMID: 35369798 Free PMC article.
References
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

