Chiral Recognition of Homochiral Poly (amidoamine) Dendrimers Substituted with R- and S-Glycidol by Keratinocyte (HaCaT) and Squamous Carcinoma (SCC-15) Cells In Vitro
- PMID: 33801610
- PMCID: PMC8037736
- DOI: 10.3390/polym13071049
Chiral Recognition of Homochiral Poly (amidoamine) Dendrimers Substituted with R- and S-Glycidol by Keratinocyte (HaCaT) and Squamous Carcinoma (SCC-15) Cells In Vitro
Abstract
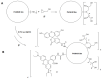
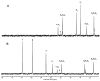
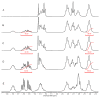
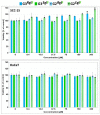
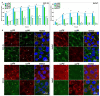
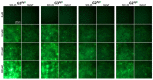
The generation 2 and 3 poly(amidoamine) dendrimers (PAMAM G2 and G3) were converted into N-(2,3-dihydroxy)propyl derivatives by the addition of enantiomerically pure S- and R-glycidol. The homochiral dendrimers bind to HaCaT and SCC-15 cell membranes with an R/S glycidol enantioselectivity ratio of 1.5:1, as was quantitatively determined by fluorescence microscopy and visualized by confocal microscopy. Fully substituted G2 and G3 dendrimers were equipped with 32 and 64 N-(2,3-dihydroxy)propyl residues and showed effectively radial symmetry for homochiral derivatives in 13C NMR spectrum in contrary to analogs obtained by reaction with rac-glycidol. The sub-stoichiometric derivatives of G2 and G3 were also obtained in order to characterize them spectroscopically. The homochiral dendrimers were labeled with two different fluorescent labels, fluorescein, and rhodamine B, using their isothiocyanates to react with G2 and G3 followed by the addition of S- and R-glycidol. Obtained fluorescent derivatives were deficiently filled with N-(2,3-dihydroxy)propyl substituents due to steric hindrance imposed by the attached label. Nevertheless, these derivatives were used to determine their ability to bind to the cell membrane of human keratinocytes (HaCaT) and squamous carcinoma cells (SCC-15). Confocal microscopy images obtained from cells treated with variously labeled conjugates and fluorescence analysis with fluorescence reader allowed us to conclude that R-glycidol derivatives were bound and entered the cells preferentially, with higher accumulation in cancer cells. The G3 polyamidoamine (PAMAM)-based dendrimers were taken up more efficiently than G2 derivatives. Moreover, S- and R-glycidol furnished dendrimers were highly biocompatible with no toxicity up to 300 µM concentrations, in contrast to the amine-terminated PAMAM analogs.
Keywords: chiral biorecognition; confocal microscopy; glycidol; homochiral dendrimer; polyamidoamine dendrimer; toxicity.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Synthesis of Biotinylated PAMAM G3 Dendrimers Substituted with R-Glycidol and Celecoxib/Simvastatin as Repurposed Drugs and Evaluation of Their Increased Additive Cytotoxicity for Cancer Cell Lines.Cancers (Basel). 2022 Jan 29;14(3):714. doi: 10.3390/cancers14030714. Cancers (Basel). 2022. PMID: 35158983 Free PMC article.
-
Cellular uptake of glucoheptoamidated poly(amidoamine) PAMAM G3 dendrimer with amide-conjugated biotin, a potential carrier of anticancer drugs.Bioorg Med Chem. 2017 Jan 15;25(2):706-713. doi: 10.1016/j.bmc.2016.11.047. Epub 2016 Nov 25. Bioorg Med Chem. 2017. PMID: 27919613
-
Mixed-Generation PAMAM G3-G0 Megamer as a Drug Delivery System for Nimesulide: Antitumor Activity of the Conjugate Against Human Squamous Carcinoma and Glioblastoma Cells.Int J Mol Sci. 2019 Oct 9;20(20):4998. doi: 10.3390/ijms20204998. Int J Mol Sci. 2019. PMID: 31601050 Free PMC article.
-
A Novel PAMAM G3 Dendrimer-Based Foam with Polyether Polyol and Castor Oil Components as Drug Delivery System into Cancer and Normal Cells.Materials (Basel). 2024 Aug 7;17(16):3905. doi: 10.3390/ma17163905. Materials (Basel). 2024. PMID: 39203083 Free PMC article.
-
Biotin Transport-Targeting Polysaccharide-Modified PAMAM G3 Dendrimer as System Delivering α-Mangostin into Cancer Cells and C. elegans Worms.Int J Mol Sci. 2021 Nov 29;22(23):12925. doi: 10.3390/ijms222312925. Int J Mol Sci. 2021. PMID: 34884739 Free PMC article.
Cited by
-
Safety Challenges and Application Strategies for the Use of Dendrimers in Medicine.Pharmaceutics. 2022 Jun 17;14(6):1292. doi: 10.3390/pharmaceutics14061292. Pharmaceutics. 2022. PMID: 35745863 Free PMC article. Review.
-
Synthesis of Functional Building Blocks for Type III-B Rotaxane Dendrimer.Polymers (Basel). 2021 Nov 12;13(22):3909. doi: 10.3390/polym13223909. Polymers (Basel). 2021. PMID: 34833208 Free PMC article.
-
Human Embryonic Kidney HEK293 Cells as a Model to Study SMVT-Independent Transport of Biotin and Biotin-Furnished Nanoparticles in Targeted Therapy.Int J Mol Sci. 2025 Feb 13;26(4):1594. doi: 10.3390/ijms26041594. Int J Mol Sci. 2025. PMID: 40004058 Free PMC article.
-
Synthesis of Biotinylated PAMAM G3 Dendrimers Substituted with R-Glycidol and Celecoxib/Simvastatin as Repurposed Drugs and Evaluation of Their Increased Additive Cytotoxicity for Cancer Cell Lines.Cancers (Basel). 2022 Jan 29;14(3):714. doi: 10.3390/cancers14030714. Cancers (Basel). 2022. PMID: 35158983 Free PMC article.
-
The Importance of Biotinylation for the Suitability of Cationic and Neutral Fourth-Generation Polyamidoamine Dendrimers as Targeted Drug Carriers in the Therapy of Glioma and Liver Cancer.Molecules. 2024 Sep 10;29(18):4293. doi: 10.3390/molecules29184293. Molecules. 2024. PMID: 39339289 Free PMC article.
References
-
- Tomalia D.A., Baker H., Dewald J., Hall M., Kallos G., Martin S., Roeck J., Ryder J., Smith P. A New Class of Polymers: Starburst-Dendritic Macromolecules. Polym. J. 1985;17:117–132. doi: 10.1295/polymj.17.117. - DOI
-
- Tomalia D., Huang B., Swanson D., Brothers H., Klimash J. Structure Control within Poly(Amidoamine) Dendrimers: Size, Shape and Regio-Chemical Mimicry of Globular Proteins. Tetrahedron. 2003;59:3799–3813. doi: 10.1016/S0040-4020(03)00430-7. - DOI
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

