Practical Synthesis of 2-Iodosobenzoic Acid (IBA) without Contamination by Hazardous 2-Iodoxybenzoic Acid (IBX) under Mild Conditions
- PMID: 33801611
- PMCID: PMC8036297
- DOI: 10.3390/molecules26071897
Practical Synthesis of 2-Iodosobenzoic Acid (IBA) without Contamination by Hazardous 2-Iodoxybenzoic Acid (IBX) under Mild Conditions
Abstract
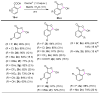
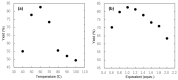
We report a convenient and practical method for the preparation of nonexplosive cyclic hypervalent iodine(III) oxidants as efficient organocatalysts and reagents for various reactions using Oxone® in aqueous solution under mild conditions at room temperature. The thus obtained 2-iodosobenzoic acids (IBAs) could be used as precursors of other cyclic organoiodine(III) derivatives by the solvolytic derivatization of the hydroxy group under mild conditions of 80 °C or lower temperature. These sequential procedures are highly reliable to selectively afford cyclic hypervalent iodine compounds in excellent yields without contamination by hazardous pentavalent iodine(III) compound.
Keywords: Oxone®; cyclic organoiodine(III) compounds; water, solvolytic functionalization, mild condition, metal-free, 2-iodosobenzoic acid.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
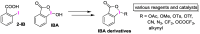






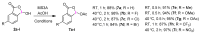
Similar articles
-
In situ generation of o-iodoxybenzoic acid (IBX) and the catalytic use of it in oxidation reactions in the presence of Oxone as a co-oxidant.Org Lett. 2005 Jul 7;7(14):2933-6. doi: 10.1021/ol050875o. Org Lett. 2005. PMID: 15987173
-
2-Iodoxybenzoic acid organosulfonates: preparation, X-ray structure and reactivity of new, powerful hypervalent iodine(V) oxidants.Chem Commun (Camb). 2013 Dec 14;49(96):11269-71. doi: 10.1039/c3cc47090c. Epub 2013 Oct 24. Chem Commun (Camb). 2013. PMID: 24153437
-
2-Iodoxybenzenesulfonic acid as an extremely active catalyst for the selective oxidation of alcohols to aldehydes, ketones, carboxylic acids, and enones with oxone.J Am Chem Soc. 2009 Jan 14;131(1):251-62. doi: 10.1021/ja807110n. J Am Chem Soc. 2009. PMID: 19053813
-
Hypervalent-Iodine(III)-Mediated Oxidative Methodology for the Synthesis of Fused Triazoles.Chem Asian J. 2016 Jul 20;11(14):1988-2000. doi: 10.1002/asia.201600119. Epub 2016 Jun 6. Chem Asian J. 2016. PMID: 27123538 Review.
-
Iodine(V)-Based Oxidants in Oxidation Reactions.Molecules. 2023 Jul 6;28(13):5250. doi: 10.3390/molecules28135250. Molecules. 2023. PMID: 37446912 Free PMC article. Review.
References
LinkOut - more resources
Full Text Sources
Other Literature Sources

