Evolution of Multicellular Complexity in The Dictyostelid Social Amoebas
- PMID: 33801615
- PMCID: PMC8067170
- DOI: 10.3390/genes12040487
Evolution of Multicellular Complexity in The Dictyostelid Social Amoebas
Abstract
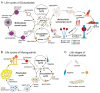
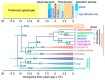
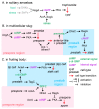
Multicellularity evolved repeatedly in the history of life, but how it unfolded varies greatly between different lineages. Dictyostelid social amoebas offer a good system to study the evolution of multicellular complexity, with a well-resolved phylogeny and molecular genetic tools being available. We compare the life cycles of the Dictyostelids with closely related amoebozoans to show that complex life cycles were already present in the unicellular common ancestor of Dictyostelids. We propose frost resistance as an early driver of multicellular evolution in Dictyostelids and show that the cell signalling pathways for differentiating spore and stalk cells evolved from that for encystation. The stalk cell differentiation program was further modified, possibly through gene duplication, to evolve a new cell type, cup cells, in Group 4 Dictyostelids. Studies in various multicellular organisms, including Dictyostelids, volvocine algae, and metazoans, suggest as a common principle in the evolution of multicellular complexity that unicellular regulatory programs for adapting to environmental change serve as "proto-cell types" for subsequent evolution of multicellular organisms. Later, new cell types could further evolve by duplicating and diversifying the "proto-cell type" gene regulatory networks.
Keywords: amoebozoa; cAMP signalling; cell type evolution; dictyostelia; encystation; evolution of multicellularity.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- El Albani A., Bengtson S., Canfield D.E., Bekker A., Macchiarelli R., Mazurier A., Hammarlund E.U., Boulvais P., Dupuy J.J., Fontaine C., et al. Large colonial organisms with coordinated growth in oxygenated environments 2.1 Gyr ago. Nature. 2010;466:100–104. doi: 10.1038/nature09166. - DOI - PubMed
-
- El Albani A., Mangano M.G., Buatois L.A., Bengtson S., Riboulleau A., Bekker A., Konhauser K., Lyons T., Rollion-Bard C., Bankole O., et al. Organism motility in an oxygenated shallow-marine environment 2.1 billion years ago. Proc. Natl. Acad. Sci. USA. 2019;116:3431–3436. doi: 10.1073/pnas.1815721116. - DOI - PMC - PubMed
-
- Grosberg R.K., Strathmann R.R. The evolution of multicellularity: A minor major transition? Annu. Rev. Ecol. Evol. Syst. 2007;38:621–654. doi: 10.1146/annurev.ecolsys.36.102403.114735. - DOI
-
- Knoll A.H. The Multiple Origins of Complex Multicellularity. Annu. Rev. Earth Planet. Sci. 2011;39:217–239. doi: 10.1146/annurev.earth.031208.100209. - DOI
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

