Pineal Gland Tumors: A Review
- PMID: 33801639
- PMCID: PMC8036741
- DOI: 10.3390/cancers13071547
Pineal Gland Tumors: A Review
Abstract
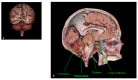
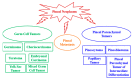
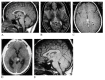
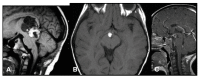
The pineal gland is a small, pinecone-shaped endocrine gland that participates in the biological rhythm regulation of vertebrates. The recognized major product of the pineal gland is melatonin-a multifunctional endogenous indoleamine. Accumulating evidence suggests that the pineal gland is important for preserving ideal health conditions in vertebrate. Tumors of the pineal region account for approximately 3-11% of pediatric brain neoplasms but fewer than 1% of brain neoplasms in adults. It is fundamental to expand advanced imaging techniques together with both clinical and laboratory knowledge, to help to differentiate among pineal neoplasms and thus facilitate accurate primary diagnoses and proper therapeutic interventions. In this review, we report the gross anatomy of the pineal gland and its functional significance and discuss the clinical relevance of pineal gland tumors, underlining the importance of identifying the leading causes of pineal region masses.
Keywords: brain neoplasms; pineal germ cell tumors; pineal gland; pineal metastasis; pineal parenchymal tumor.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




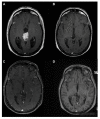

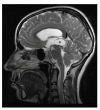
References
-
- Moretti R., Zanin A., Pansiot J., Spiri D., Manganozzi L., Kratzer I., Favero G., Vasiljevic A., Rinaldi V.E., Pic I., et al. Melatonin reduces excitotoxic blood-brain barrier breakdown in neonatal rats. Neuroscience. 2015;311:382–397. doi: 10.1016/j.neuroscience.2015.10.044. Erratum in: Neuroscience2016, 315, 296. - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

