Overexpression of Human Syndecan-1 Protects against the Diethylnitrosamine-Induced Hepatocarcinogenesis in Mice
- PMID: 33801718
- PMCID: PMC8037268
- DOI: 10.3390/cancers13071548
Overexpression of Human Syndecan-1 Protects against the Diethylnitrosamine-Induced Hepatocarcinogenesis in Mice
Abstract
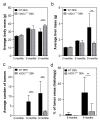
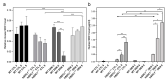
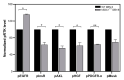
Although syndecan-1 (SDC1) is known to be dysregulated in various cancer types, its implication in tumorigenesis is poorly understood. Its effect may be detrimental or protective depending on the type of cancer. Our previous data suggest that SDC1 is protective against hepatocarcinogenesis. To further verify this notion, human SDC1 transgenic (hSDC1+/+) mice were generated that expressed hSDC1 specifically in the liver under the control of the albumin promoter. Hepatocarcinogenesis was induced by a single dose of diethylnitrosamine (DEN) at an age of 15 days after birth, which resulted in tumors without cirrhosis in wild-type and hSDC1+/+ mice. At the experimental endpoint, livers were examined macroscopically and histologically, as well as by immunohistochemistry, Western blot, receptor tyrosine kinase array, phosphoprotein array, and proteomic analysis. Liver-specific overexpression of hSDC1 resulted in an approximately six month delay in tumor formation via the promotion of SDC1 shedding, downregulation of lipid metabolism, inhibition of the mTOR and the β-catenin pathways, and activation of the Foxo1 and p53 transcription factors that lead to the upregulation of the cell cycle inhibitors p21 and p27. Furthermore, both of them are implicated in the regulation of intermediary metabolism. Proteomic analysis showed enhanced lipid metabolism, activation of motor proteins, and loss of mitochondrial electron transport proteins as promoters of cancer in wild-type tumors, inhibited in the hSDC1+/+ livers. These complex mechanisms mimic the characteristics of nonalcoholic steatohepatitis (NASH) induced human liver cancer successfully delayed by syndecan-1.
Keywords: lipid metabolism; liver carcinogenesis; mouse; syndecan-1.
Conflict of interest statement
The author (L.S.) is the founder of Szilak Laboratories Bioinformatics and Molecule-Design Ltd. This founder provided the syndecan-1 constructs taking part in the design of the study. The author (P.T.) is employed by Solvo Biotechnology. The funder (Solvo Biotechnology) provided support in the form of author’s salaries for the author (P.T.), but did not have any additional role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results. The remaining authors declare no conflict of interest.
Figures








Similar articles
-
Syndecan-1 inhibits early stages of liver fibrogenesis by interfering with TGFβ1 action and upregulating MMP14.Matrix Biol. 2018 Aug;68-69:474-489. doi: 10.1016/j.matbio.2018.02.008. Epub 2018 Feb 16. Matrix Biol. 2018. PMID: 29454902
-
Overexpression of glutamine synthetase is associated with beta-catenin-mutations in mouse liver tumors during promotion of hepatocarcinogenesis by phenobarbital.Cancer Res. 2002 Oct 15;62(20):5685-8. Cancer Res. 2002. PMID: 12384525
-
Role of the BrafV637E mutation in hepatocarcinogenesis induced by treatment with diethylnitrosamine in neonatal B6C3F1 mice.Mol Carcinog. 2017 Feb;56(2):478-488. doi: 10.1002/mc.22510. Epub 2016 Jun 10. Mol Carcinog. 2017. PMID: 27253992 Free PMC article.
-
Mammary gland development requires syndecan-1 to create a beta-catenin/TCF-responsive mammary epithelial subpopulation.Oncogene. 2003 Dec 18;22(58):9243-53. doi: 10.1038/sj.onc.1207217. Oncogene. 2003. PMID: 14681683
-
Tumor formation in liver of conditional β-catenin-deficient mice exposed to a diethylnitrosamine/phenobarbital tumor promotion regimen.Carcinogenesis. 2011 Jan;32(1):52-7. doi: 10.1093/carcin/bgq226. Epub 2010 Nov 3. Carcinogenesis. 2011. PMID: 21047994
Cited by
-
Matrix Effectors and Cancer.Cancers (Basel). 2021 Dec 31;14(1):200. doi: 10.3390/cancers14010200. Cancers (Basel). 2021. PMID: 35008364 Free PMC article.
-
Expression of glycosaminoglycans in cirrhotic liver and hepatocellular carcinoma-a pilot study including etiology.Anal Bioanal Chem. 2022 May;414(13):3837-3846. doi: 10.1007/s00216-022-04025-3. Epub 2022 Mar 28. Anal Bioanal Chem. 2022. PMID: 35344068 Free PMC article.
-
Proteoglycans are protagonists in autophagy, lymphangiogenesis, and neurodegenerative diseases.Trends Cell Biol. 2025 Jul 10:S0962-8924(25)00127-8. doi: 10.1016/j.tcb.2025.06.002. Online ahead of print. Trends Cell Biol. 2025. PMID: 40645813 Review.
-
Development and Validation of a Prognostic Classifier Based on Lipid Metabolism-Related Genes for Breast Cancer.J Inflamm Res. 2022 Jun 14;15:3477-3499. doi: 10.2147/JIR.S357144. eCollection 2022. J Inflamm Res. 2022. PMID: 35726216 Free PMC article.
-
SPOCK1 Promotes the Development of Hepatocellular Carcinoma.Front Oncol. 2022 Feb 3;12:819883. doi: 10.3389/fonc.2022.819883. eCollection 2022. Front Oncol. 2022. PMID: 35186754 Free PMC article.
References
-
- Lambaerts K., Van Dyck S., Mortier E., Ivarsson Y., Degeest G., Luyten A., Vermeiren E., Peers B., David G., Zimmermann P. Syntenin, a syndecan adaptor and an Arf6 phosphatidylinositol 4,5-bisphosphate effector, is essential for epiboly and gastrulation cell movements in zebrafish. J. Cell Sci. 2012;125:1129–1140. doi: 10.1242/jcs.089987. - DOI - PMC - PubMed
-
- Gopal S., Søgaard P., Multhaupt H.A., Pataki C., Okina E., Xian X., Pedersen M.E., Stevens T., Griesbeck O., Park P.W., et al. Transmembrane proteoglycans control stretch-activated channels to set cytosolic calcium levels. J. Cell Biol. 2015;210:1199–1211. doi: 10.1083/jcb.201501060. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

