Diagnosis of Pancreatic Ductal Adenocarcinoma by Immuno-Positron Emission Tomography
- PMID: 33801810
- PMCID: PMC8000738
- DOI: 10.3390/jcm10061151
Diagnosis of Pancreatic Ductal Adenocarcinoma by Immuno-Positron Emission Tomography
Abstract
Diagnosis of pancreatic ductal adenocarcinoma (PDAC) by current imaging techniques is useful and widely used in the clinic but presents several limitations and challenges, especially in small lesions that frequently cause radiological tumors infra-staging, false-positive diagnosis of metastatic tumor recurrence, and common occult micro-metastatic disease. The revolution in cancer multi-"omics" and bioinformatics has uncovered clinically relevant alterations in PDAC that still need to be integrated into patients' clinical management, urging the development of non-invasive imaging techniques against principal biomarkers to assess and incorporate this information into the clinical practice. "Immuno-PET" merges the high target selectivity and specificity of antibodies and engineered fragments toward a given tumor cell surface marker with the high spatial resolution, sensitivity, and quantitative capabilities of positron emission tomography (PET) imaging techniques. In this review, we detail and provide examples of the clinical limitations of current imaging techniques for diagnosing PDAC. Furthermore, we define the different components of immuno-PET and summarize the existing applications of this technique in PDAC. The development of novel immuno-PET methods will make it possible to conduct the non-invasive diagnosis and monitoring of patients over time using in vivo, integrated, quantifiable, 3D, whole body immunohistochemistry working like a "virtual biopsy".
Keywords: PDAC; diagnostic imaging; immuno-PET; pancreatic cancer.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
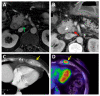
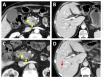
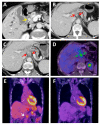

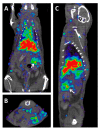
Similar articles
-
Diagnosis of Glioblastoma by Immuno-Positron Emission Tomography.Cancers (Basel). 2021 Dec 24;14(1):74. doi: 10.3390/cancers14010074. Cancers (Basel). 2021. PMID: 35008238 Free PMC article. Review.
-
More advantages in detecting bone and soft tissue metastases from prostate cancer using 18F-PSMA PET/CT.Hell J Nucl Med. 2019 Jan-Apr;22(1):6-9. doi: 10.1967/s002449910952. Epub 2019 Mar 7. Hell J Nucl Med. 2019. PMID: 30843003
-
Dual-Modality Immuno-PET and Near-Infrared Fluorescence Imaging of Pancreatic Cancer Using an Anti-Prostate Stem Cell Antigen Cys-Diabody.J Nucl Med. 2018 Sep;59(9):1398-1405. doi: 10.2967/jnumed.117.207332. Epub 2018 Mar 30. J Nucl Med. 2018. PMID: 29602820 Free PMC article.
-
The Role of 18F-FDG PET/CT and PET/MRI in Pancreatic Ductal Adenocarcinoma.Abdom Radiol (NY). 2018 Feb;43(2):415-434. doi: 10.1007/s00261-017-1374-2. Abdom Radiol (NY). 2018. PMID: 29143875 Review.
-
Impact of 68Ga-FAPI PET/CT Imaging on the Therapeutic Management of Primary and Recurrent Pancreatic Ductal Adenocarcinomas.J Nucl Med. 2021 Jun 1;62(6):779-786. doi: 10.2967/jnumed.120.253062. Epub 2020 Oct 23. J Nucl Med. 2021. PMID: 33097632 Free PMC article.
Cited by
-
ImmunoPET: Antibody-Based PET Imaging in Solid Tumors.Front Med (Lausanne). 2022 Jun 28;9:916693. doi: 10.3389/fmed.2022.916693. eCollection 2022. Front Med (Lausanne). 2022. PMID: 35836956 Free PMC article. Review.
-
Synthesis and In Vivo Evaluation of a Site-specifically Labeled Radioimmunoconjugate for Dual-Modal (PET/NIRF) Imaging of MT1-MMP in Sarcomas.Bioconjug Chem. 2022 Aug 17;33(8):1564-1573. doi: 10.1021/acs.bioconjchem.2c00306. Epub 2022 Jul 22. Bioconjug Chem. 2022. PMID: 35867034 Free PMC article.
-
Application of Proteomics in Pancreatic Ductal Adenocarcinoma Biomarker Investigations: A Review.Int J Mol Sci. 2022 Feb 14;23(4):2093. doi: 10.3390/ijms23042093. Int J Mol Sci. 2022. PMID: 35216204 Free PMC article. Review.
-
Automated classification of urine biomarkers to diagnose pancreatic cancer using 1-D convolutional neural networks.J Biol Eng. 2023 Apr 17;17(1):28. doi: 10.1186/s13036-023-00340-0. J Biol Eng. 2023. PMID: 37069681 Free PMC article.
-
Radiopharmaceuticals for Pancreatic Cancer: A Review of Current Approaches and Future Directions.Pharmaceuticals (Basel). 2024 Oct 1;17(10):1314. doi: 10.3390/ph17101314. Pharmaceuticals (Basel). 2024. PMID: 39458955 Free PMC article. Review.
References
-
- Gómez M.C., Sabater L., Ferrández A. Protocolo detallado, estudio e informe anatomopatológico de las piezas de duodenopancreatectomía cefálica por carcinoma de páncreas. Rev. Esp. Patol. 2010;43:207–214. doi: 10.1016/j.patol.2010.07.002. - DOI
-
- Altekruse S.F., Kosary C.L., Krapcho M., Neyman N., Aminou R., Waldron W., Ruhl J., Howlader N., Tatalovich Z., Cho H., et al. SEER Cancer Statistics Review, 1975–2007. [(accessed on 23 December 2020)];Natl. Cancer Instit. 2010 Available online: https://seer.cancer.gov/csr/1975_2007/
-
- Cassinotto C., Cortade J., Belleannée G., Lapuyade B., Terrebonne E., Vendrely V., Laurent C., Sa-Cunha A. An evaluation of the accuracy of CT when determining resectability of pancreatic head adenocarcinoma after neoadjuvant treatment. Eur. J. Radiol. 2013;82:589–593. doi: 10.1016/j.ejrad.2012.12.002. - DOI - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

