Linking Serine/Glycine Metabolism to Radiotherapy Resistance
- PMID: 33801846
- PMCID: PMC8002185
- DOI: 10.3390/cancers13061191
Linking Serine/Glycine Metabolism to Radiotherapy Resistance
Abstract
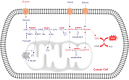
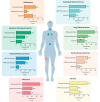
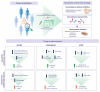
The activation of de novo serine/glycine biosynthesis in a subset of tumors has been described as a major contributor to tumor pathogenesis, poor outcome, and treatment resistance. Amplifications and mutations of de novo serine/glycine biosynthesis enzymes can trigger pathway activation; however, a large group of cancers displays serine/glycine pathway overexpression induced by oncogenic drivers and unknown regulatory mechanisms. A better understanding of the regulatory network of de novo serine/glycine biosynthesis activation in cancer might be essential to unveil opportunities to target tumor heterogeneity and therapy resistance. In the current review, we describe how the activation of de novo serine/glycine biosynthesis in cancer is linked to treatment resistance and its implications in the clinic. To our knowledge, only a few studies have identified this pathway as metabolic reprogramming of cancer cells in response to radiation therapy. We propose an important contribution of de novo serine/glycine biosynthesis pathway activation to radioresistance by being involved in cancer cell viability and proliferation, maintenance of cancer stem cells (CSCs), and redox homeostasis under hypoxia and nutrient-deprived conditions. Current approaches for inhibition of the de novo serine/glycine biosynthesis pathway provide new opportunities for therapeutic intervention, which in combination with radiotherapy might be a promising strategy for tumor control and ultimately eradication. Further research is needed to gain molecular and mechanistic insight into the activation of this pathway in response to radiation therapy and to design sophisticated stratification methods to select patients that might benefit from serine/glycine metabolism-targeted therapies in combination with radiotherapy.
Keywords: DNA repair; PHGDH; PSAT1; PSPH; SHMT; cancer; hypoxia; radiotherapy; redox homeostasis; resistance; serine and glycine metabolism.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
AMPK-HIF-1α signaling enhances glucose-derived de novo serine biosynthesis to promote glioblastoma growth.J Exp Clin Cancer Res. 2023 Dec 15;42(1):340. doi: 10.1186/s13046-023-02927-3. J Exp Clin Cancer Res. 2023. PMID: 38098117 Free PMC article.
-
Bioinformatics analysis of the serine and glycine pathway in cancer cells.Oncotarget. 2014 Nov 30;5(22):11004-13. doi: 10.18632/oncotarget.2668. Oncotarget. 2014. PMID: 25436979 Free PMC article.
-
The role of serine metabolism in lung cancer: From oncogenesis to tumor treatment.Front Genet. 2023 Jan 9;13:1084609. doi: 10.3389/fgene.2022.1084609. eCollection 2022. Front Genet. 2023. PMID: 36699468 Free PMC article. Review.
-
Menin regulates the serine biosynthetic pathway in Ewing sarcoma.J Pathol. 2018 Jul;245(3):324-336. doi: 10.1002/path.5085. Epub 2018 May 28. J Pathol. 2018. PMID: 29672864 Free PMC article.
-
An essential role for de novo biosynthesis of L-serine in CNS development.Asia Pac J Clin Nutr. 2008;17 Suppl 1:312-5. Asia Pac J Clin Nutr. 2008. PMID: 18296366 Review.
Cited by
-
Unraveling the role of the mitochondrial one-carbon pathway in undifferentiated thyroid cancer by multi-omics analyses.Nat Commun. 2024 Feb 8;15(1):1163. doi: 10.1038/s41467-024-45366-0. Nat Commun. 2024. PMID: 38331894 Free PMC article.
-
Inhibition of Phosphoglycerate Dehydrogenase Radiosensitizes Human Colorectal Cancer Cells under Hypoxic Conditions.Cancers (Basel). 2022 Oct 15;14(20):5060. doi: 10.3390/cancers14205060. Cancers (Basel). 2022. PMID: 36291844 Free PMC article.
-
Targeting serine/glycine metabolism improves radiotherapy response in non-small cell lung cancer.Br J Cancer. 2024 Mar;130(4):568-584. doi: 10.1038/s41416-023-02553-y. Epub 2023 Dec 30. Br J Cancer. 2024. PMID: 38160212 Free PMC article.
-
Glioblastomas: Hijacking Metabolism to Build a Flexible Shield for Therapy Resistance.Antioxid Redox Signal. 2023 Nov;39(13-15):957-979. doi: 10.1089/ars.2022.0088. Epub 2023 Apr 5. Antioxid Redox Signal. 2023. PMID: 37022791 Free PMC article. Review.
-
Multi-omics Analysis of the Role of PHGDH in Colon Cancer.Technol Cancer Res Treat. 2023 Jan-Dec;22:15330338221145994. doi: 10.1177/15330338221145994. Technol Cancer Res Treat. 2023. PMID: 36707056 Free PMC article.
References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

