Resistance of Hypoxic Cells to Ionizing Radiation Is Mediated in Part via Hypoxia-Induced Quiescence
- PMID: 33801903
- PMCID: PMC7998378
- DOI: 10.3390/cells10030610
Resistance of Hypoxic Cells to Ionizing Radiation Is Mediated in Part via Hypoxia-Induced Quiescence
Abstract
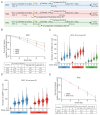
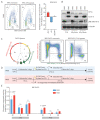
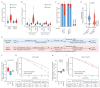
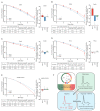
Double strand breaks (DSBs) are highly toxic to a cell, a property that is exploited in radiation therapy. A critical component for the damage induction is cellular oxygen, making hypoxic tumor areas refractory to the efficacy of radiation treatment. During a fractionated radiation regimen, these hypoxic areas can be re-oxygenated. Nonetheless, hypoxia still constitutes a negative prognostic factor for the patient's outcome. We hypothesized that this might be attributed to specific hypoxia-induced cellular traits that are maintained upon reoxygenation. Here, we show that reoxygenation of hypoxic non-transformed RPE-1 cells fully restored induction of DSBs but the cells remain radioresistant as a consequence of hypoxia-induced quiescence. With the use of the cell cycle indicators (FUCCI), cell cycle-specific radiation sensitivity, the cell cycle phase duration with live cell imaging, and single cell tracing were assessed. We observed that RPE-1 cells experience a longer G1 phase under hypoxia and retain a large fraction of cells that are non-cycling. Expression of HPV oncoprotein E7 prevents hypoxia-induced quiescence and abolishes the radioprotective effect. In line with this, HPV-negative cancer cell lines retain radioresistance, while HPV-positive cancer cell lines are radiosensitized upon reoxygenation. Quiescence induction in hypoxia and its HPV-driven prevention was observed in 3D multicellular spheroids. Collectively, we identify a new hypoxia-dependent radioprotective phenotype due to hypoxia-induced quiescence that accounts for a global decrease in radiosensitivity that can be retained upon reoxygenation and is absent in cells expressing oncoprotein E7.
Keywords: G1-arrest; HPV; hypoxia; quiescence; radiation resistance.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

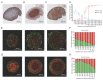
References
-
- Lalonde E., Ishkanian A.S., Sykes J., Fraser M., Ross-Adams H., Erho N., Dunning M.J., Halim S., Lamb A.D., Moon N.C., et al. Tumour genomic and microenvironmental heterogeneity for integrated prediction of 5-year biochemical recurrence of prostate cancer: A retrospective cohort study. Lancet Oncol. 2014;15:1521–1532. doi: 10.1016/S1470-2045(14)71021-6. - DOI - PubMed
-
- Linge A., Lohaus F., Löck S., Nowak A., Gudziol V., Valentini C., Von Neubeck C., Jütz M., Tinhofer I., Budach V., et al. HPV status, cancer stem cell marker expression, hypoxia gene signatures and tumour volume identify good prognosis subgroups in patients with HNSCC after primary radiochemotherapy: A multicentre retrospective study of the German Cancer Consortium Radiation Oncology Group (DKTK-ROG) Radiother. Oncol. 2016;121:364–373. doi: 10.1016/j.radonc.2016.11.008. - DOI - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

