Immunogenicity of HIV-1-Based Virus-Like Particles with Increased Incorporation and Stability of Membrane-Bound Env
- PMID: 33801906
- PMCID: PMC8002006
- DOI: 10.3390/vaccines9030239
Immunogenicity of HIV-1-Based Virus-Like Particles with Increased Incorporation and Stability of Membrane-Bound Env
Abstract
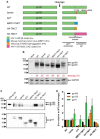
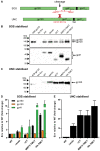
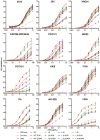
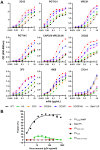
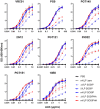
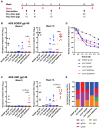
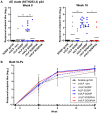
An optimal prophylactic vaccine to prevent human immunodeficiency virus (HIV-1) transmission should elicit protective antibody responses against the HIV-1 envelope glycoprotein (Env). Replication-incompetent HIV-1 virus-like particles (VLPs) offer the opportunity to present virion-associated Env with a native-like structure during vaccination that closely resembles that encountered on infectious virus. Here, we optimized the incorporation of Env into previously designed mature-form VLPs (mVLPs) and assessed their immunogenicity in mice. The incorporation of Env into mVLPs was increased by replacing the Env transmembrane and cytoplasmic tail domains with those of influenza haemagglutinin (HA-TMCT). Furthermore, Env was stabilized on the VLP surface by introducing an interchain disulfide and proline substitution (SOSIP) mutations typically employed to stabilize soluble Env trimers. The resulting mVLPs efficiently presented neutralizing antibody epitopes while minimizing exposure of non-neutralizing antibody sites. Vaccination of mice with mVLPs elicited a broader range of Env-specific antibody isotypes than Env presented on immature VLPs or extracellular vesicles. The mVLPs bearing HA-TMCT-modified Env consistently induced anti-Env antibody responses that mediated modest neutralization activity. These mVLPs are potentially useful immunogens for eliciting neutralizing antibody responses that target native Env epitopes on infectious HIV-1 virions.
Keywords: Env incorporation; HIV-1; SOSIP; VLP; mature form; virus-like particle.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures
References
LinkOut - more resources
Full Text Sources
Other Literature Sources

