The Impact of Polyamine Precursors, Polyamines, and Steroid Hormones on Temporal Messenger RNA Abundance in Bovine Satellite Cells Induced to Differentiate
- PMID: 33801966
- PMCID: PMC8001141
- DOI: 10.3390/ani11030764
The Impact of Polyamine Precursors, Polyamines, and Steroid Hormones on Temporal Messenger RNA Abundance in Bovine Satellite Cells Induced to Differentiate
Abstract
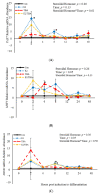
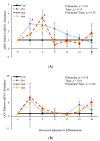
Emerging research suggests that hormones found in anabolic implants interact with polyamine biosynthesis. The objective of this study was to determine the effects of steroidal hormones, polyamines and polyamine precursors on bovine satellite cell (BSC) differentiation and polyamine biosynthesis temporally. Primary BSCs were induced to differentiate in 3% horse serum (CON) and treated with 10 nM trenbolone acetate (TBA), 10 nM estradiol (E2), 10 nM TBA and 10 nM E2, 10 mM methionine, 8 mM ornithine, 2 mM putrescine, 1.5 mM spermidine, or 0.5 mM spermine. Total mRNA was isolated 0, 2, 4, 8, 12, 24, and 48 h post-treatment. Abundance of mRNA for genes associated with induction of BSC differentiation: paired box transcription factor 7, myogenic factor 5, and myogenic differentiation factor 1 and genes in the polyamine biosynthesis pathway: ornithine decarboxylase and S-adenosylmethionine-were analyzed. Overall, steroidal hormones did not impact (p > 0.05) mRNA abundance of genes involved in BSC differentiation, but did alter (p = 0.04) abundance of genes involved in polyamine biosynthesis. Polyamine precursors influenced (p < 0.05) mRNA of genes involved in BSC differentiation. These results indicate that polyamine precursors and polyamines impact BSC differentiation and abundance of mRNA involved in polyamine biosynthesis, while steroidal hormones altered the mRNA involved in polyamine biosynthesis.
Keywords: anabolic implants; beef; bovine satellite cells; polyamines; skeletal muscle.
Conflict of interest statement
The authors declare no conflict of interest.
Figures







References
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

