Tumor Activated Cell Penetrating Peptides to Selectively Deliver Immune Modulatory Drugs
- PMID: 33801967
- PMCID: PMC8000974
- DOI: 10.3390/pharmaceutics13030365
Tumor Activated Cell Penetrating Peptides to Selectively Deliver Immune Modulatory Drugs
Abstract
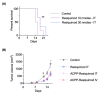
Recent advances in immunotherapy have revolutionized cancer therapy. Immunotherapies can engage the adaptive and innate arms of the immune system. Therapeutics targeting immune checkpoint inhibitors (i.e., CTLA-4; PD-1, and PD-L1) have shown efficacy for subsets of cancer patients by unleashing an adaptive antitumor immune response. Alternatively, small molecule immune modulators of the innate immune system such as toll-like receptor (TLR) agonists are being developed for cancer therapy. TLRs function as pattern recognition receptors to microbial products and are also involved in carcinogenesis. Reisquimod is a TLR 7/8 agonist that has antitumor efficacy. However, systemic delivery free resiquimod has proven to be challenging due to toxicity of nonspecific TLR 7/8 activation. Therefore, we developed a targeted peptide-drug conjugate strategy for systemic delivery of resiquimod. We designed an activatable cell penetrating peptide to deliver resiquimod specifically to the tumor tissue while avoiding normal tissues. The activatable cell penetrating peptide (ACPP) scaffold undergoes enzymatic cleavage by matrix metalloproteinases 2/9 in the extracellular matrix followed by intracellular lysosomal cathepsin B mediated release of the free resiquimod. Importantly, when conjugated to ACPP; the tumor tissue concentration of resiquimod was more than 1000-fold greater than that of surrounding non-cancerous tissue. Moreover, systemic ACPP-resiquimod delivery produced comparable therapeutic efficacy to localized free resiquimod in syngeneic murine tumors. These results highlight a precision peptide-drug conjugate delivery.
Keywords: cell penetrating peptides; matrix metalloproteinases; targeted drug delivery; toll-like receptor ligand.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





References
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

