Zeolites at the Molecular Level: What Can Be Learned from Molecular Modeling
- PMID: 33801999
- PMCID: PMC8001918
- DOI: 10.3390/molecules26061511
Zeolites at the Molecular Level: What Can Be Learned from Molecular Modeling
Abstract
This review puts the development of molecular modeling methods in the context of their applications to zeolitic active sites. We attempt to highlight the utmost necessity of close cooperation between theory and experiment, resulting both in advances in computational methods and in progress in experimental techniques.
Keywords: Brønsted and Lewis acid sites; DFT; electron density distribution; molecular modeling; multiple bond activation; transition metal sites; wave function methods; zeolite.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

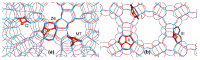
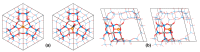
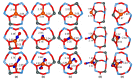
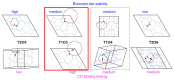

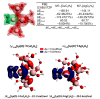

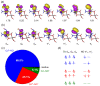
References
-
- Corma A. State of the art and future challenges of zeolites as catalysts. J. Catal. 2003;216:298–312. doi: 10.1016/S0021-9517(02)00132-X. - DOI
-
- Li Y., Li L., Yu J. Applications of zeolites in sustainable chemistry. Chem. 2017;3:928–949. doi: 10.1016/j.chempr.2017.10.009. - DOI
-
- Chizallet C. Toward the atomic scale simulation of intricate acidic aluminosilicate catalysts. ACS Catal. 2020;10:5579–5601. doi: 10.1021/acscatal.0c01136. - DOI
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

