Advances in Electrochemical Energy Devices Constructed with Tungsten Oxide-Based Nanomaterials
- PMID: 33802013
- PMCID: PMC8000231
- DOI: 10.3390/nano11030692
Advances in Electrochemical Energy Devices Constructed with Tungsten Oxide-Based Nanomaterials
Abstract
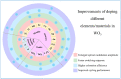
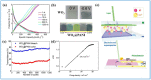
Tungsten oxide-based materials have drawn huge attention for their versatile uses to construct various energy storage devices. Particularly, their electrochromic devices and optically-changing devices are intensively studied in terms of energy-saving. Furthermore, based on close connections in the forms of device structure and working mechanisms between these two main applications, bifunctional devices of tungsten oxide-based materials with energy storage and optical change came into our view, and when solar cells are integrated, multifunctional devices are accessible. In this article, we have reviewed the latest developments of tungsten oxide-based nanostructured materials in various kinds of applications, and our focus falls on their energy-related uses, especially supercapacitors, lithium ion batteries, electrochromic devices, and their bifunctional and multifunctional devices. Additionally, other applications such as photochromic devices, sensors, and photocatalysts of tungsten oxide-based materials have also been mentioned. We hope this article can shed light on the related applications of tungsten oxide-based materials and inspire new possibilities for further uses.
Keywords: electrochromic devices; energy storage devices; multifunctional devices; tungsten oxides.
Conflict of interest statement
The authors declare no conflict of interest.
Figures















References
-
- Divya K., Østergaard J. Battery energy storage technology for power systems—An overview. Electr. Power Syst. Res. 2009;79:511–520. doi: 10.1016/j.epsr.2008.09.017. - DOI
-
- Amrouche S.O., Rekioua D., Bacha S. Overview of energy storage in renewable energy systems. Int. J. Hydrog. Energy. 2016;41:20914–20927. doi: 10.1016/j.ijhydene.2016.06.243. - DOI
-
- González A., Goikolea E., Barrena J.A., Mysyk R. Review on supercapacitors: Technologies and materials. Renew. Sustain. Energy Rev. 2016;58:1189–1206. doi: 10.1016/j.rser.2015.12.249. - DOI
-
- Baptista J.M., Sagu J.S., Kg U.W., Lobato K. State-of-the-art materials for high power and high energy supercapacitors: Performance metrics and obstacles for the transition from lab to industrial scale—A critical approach. Chem. Eng. J. 2019;374:1153–1179. doi: 10.1016/j.cej.2019.05.207. - DOI
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

