High Doses of Inactivated African Swine Fever Virus Are Safe, but Do Not Confer Protection against a Virulent Challenge
- PMID: 33802021
- PMCID: PMC7999564
- DOI: 10.3390/vaccines9030242
High Doses of Inactivated African Swine Fever Virus Are Safe, but Do Not Confer Protection against a Virulent Challenge
Abstract
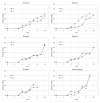
African swine fever (ASF) is currently the major concern of the global swine industry, as a consequence of which a reconsideration of the containment and prevention measures taken to date is urgently required. A great interest in developing an effective and safe vaccine against ASF virus (ASFV) infection has, therefore, recently appeared. The objective of the present study is to test an inactivated ASFV preparation under a vaccination strategy that has not previously been tested in order to improve its protective effect. The following have been considered: (i) virus inactivation by using a low binary ethyleneimine (BEI) concentration at a low temperature, (ii) the use of new and strong adjuvants; (iii) the use of very high doses (6 × 109 haemadsorption in 50% of infected cultures (HAD50)), and (iv) simultaneous double inoculation by two different routes of administration: intradermal and intramuscular. Five groups of pigs were, therefore, inoculated with BEI- Pol16/DP/OUT21 in different adjuvant formulations, twice with a 4-week interval. Six weeks later, all groups were intramuscularly challenged with 10 HAD50 of the virulent Pol16/DP/OUT21 ASFV isolate. All the animals had clinical signs and pathological findings consistent with ASF. This lack of effectiveness supports the claim that an inactivated virus strategy may not be a viable vaccine option with which to fight ASF.
Keywords: African swine fever; domestic pigs; inactivated virus; vaccine trial.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
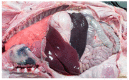
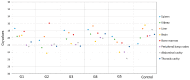
References
-
- Sánchez-Vizcaíno J.M., Laddomada A., Arias M.L. Diseases of Swine. John Wiley & Sons, Inc.; Hoboken, NJ, USA: 2019. African Swine Fever Virus; pp. 443–452.
-
- Jurado C., Martínez-Avilés M., De La Torre A., Štukelj M., de Carvalho Ferreira H.C., Cerioli M., Sánchez-Vizcaíno J.M., Bellini S. Relevant Measures to Prevent the Spread of African Swine Fever in the European Union Domestic Pig Sector. Front. Vet. Sci. 2018;5:77. doi: 10.3389/fvets.2018.00077. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

