Production of Enantiopure Chiral Epoxides with E. coli Expressing Styrene Monooxygenase
- PMID: 33802034
- PMCID: PMC8001364
- DOI: 10.3390/molecules26061514
Production of Enantiopure Chiral Epoxides with E. coli Expressing Styrene Monooxygenase
Abstract
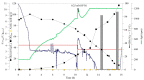
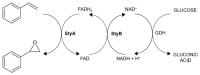
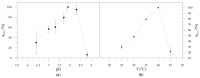
Styrene monooxygenases are a group of highly selective enzymes able to catalyse the epoxidation of alkenes to corresponding chiral epoxides in excellent enantiopurity. Chiral compounds containing oxirane ring or products of their hydrolysis represent key building blocks and precursors in organic synthesis in the pharmaceutical industry, and many of them are produced on an industrial scale. Two-component recombinant styrene monooxygenase (SMO) from Marinobacterium litorale was expressed as a fused protein (StyAL2StyB) in Escherichia coli BL21(DE3). By high cell density fermentation, 35 gDCW/L of biomass with overexpressed SMO was produced. SMO exhibited excellent stability, broad substrate specificity, and enantioselectivity, as it remained active for months and converted a group of alkenes to corresponding chiral epoxides in high enantiomeric excess (˃95-99% ee). Optically pure (S)-4-chlorostyrene oxide, (S)-allylbenzene oxide, (2R,5R)-1,2:5,6-diepoxyhexane, 2-(3-bromopropyl)oxirane, and (S)-4-(oxiran-2-yl)butan-1-ol were prepared by whole-cell SMO.
Keywords: chiral epoxides; styrene monooxygenase; whole-cell biocatalysis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Bae J.W., Park M., Jeong Y.J., Park S., Lee S.-G. Colorimetric monitoring of the activity of recombinant Escherichia coli expressing styrene monooxygenase. J. Ind. Eng. Chem. 2009;15:520–523. doi: 10.1016/j.jiec.2008.12.014. - DOI
-
- Tan C., Zhang X., Zhu Z., Xu M., Yang T., Osire T., Yang S., Rao Z. Asp305Gly mutation improved the activity and stability of the styrene monooxygenase for efficient epoxide production in Pseudomonas putida KT2440. Microb. Cell Factories. 2019;18:12. doi: 10.1186/s12934-019-1065-5. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous