Inhibitors of Cyclin-Dependent Kinases: Types and Their Mechanism of Action
- PMID: 33802080
- PMCID: PMC8001317
- DOI: 10.3390/ijms22062806
Inhibitors of Cyclin-Dependent Kinases: Types and Their Mechanism of Action
Abstract
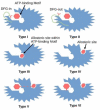
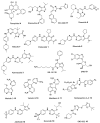
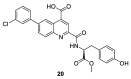
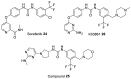
Recent studies on cyclin-dependent kinase (CDK) inhibitors have revealed that small molecule drugs have become very attractive for the treatment of cancer and neurodegenerative disorders. Most CDK inhibitors have been developed to target the ATP binding pocket. However, CDK kinases possess a very similar catalytic domain and three-dimensional structure. These features make it difficult to achieve required selectivity. Therefore, inhibitors which bind outside the ATP binding site present a great interest in the biomedical field, both from the fundamental point of view and for the wide range of their potential applications. This review tries to explain whether the ATP competitive inhibitors are still an option for future research, and highlights alternative approaches to discover more selective and potent small molecule inhibitors.
Keywords: CDK inhibitors; CDKs; cancer; cell cycle; cyclin-dependent kinase inhibitors.
Conflict of interest statement
The authors declare they have no actual or potential competing financial interests.
Figures

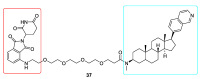
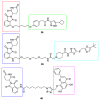
References
-
- Morgan D.O. The Cell Cycle: Principles of Control. 1st ed. New Science Press; London, UK: 2007.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

