Addressing Antiretroviral Drug Resistance with Host-Targeting Drugs-First Steps towards Developing a Host-Targeting HIV-1 Assembly Inhibitor
- PMID: 33802145
- PMCID: PMC8001593
- DOI: 10.3390/v13030451
Addressing Antiretroviral Drug Resistance with Host-Targeting Drugs-First Steps towards Developing a Host-Targeting HIV-1 Assembly Inhibitor
Abstract
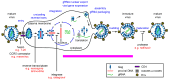
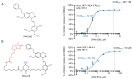
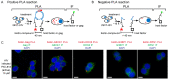
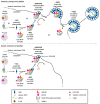
The concerning increase in HIV-1 resistance argues for prioritizing the development of host-targeting antiviral drugs because such drugs can offer high genetic barriers to the selection of drug-resistant viral variants. Targeting host proteins could also yield drugs that act on viral life cycle events that have proven elusive to inhibition, such as intracellular events of HIV-1 immature capsid assembly. Here, we review small molecule inhibitors identified primarily through HIV-1 self-assembly screens and describe how all act either narrowly post-entry or broadly on early and late events of the HIV-1 life cycle. We propose that a different screening approach could identify compounds that specifically inhibit HIV-1 Gag assembly, as was observed when a potent rabies virus inhibitor was identified using a host-catalyzed rabies assembly screen. As an example of this possibility, we discuss an antiretroviral small molecule recently identified using a screen that recapitulates the host-catalyzed HIV-1 capsid assembly pathway. This chemotype potently blocks HIV-1 replication in T cells by specifically inhibiting immature HIV-1 capsid assembly but fails to select for resistant viral variants over 37 passages, suggesting a host protein target. Development of such small molecules could yield novel host-targeting antiretroviral drugs and provide insight into chronic diseases resulting from dysregulation of host machinery targeted by these drugs.
Keywords: ABCE1; DDX6; Gag; HIV-1 assembly; HIV-1 capsid; RNA granule; antiretroviral; antiviral; drug screen; viral-host interactions.
Conflict of interest statement
The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results. J.R.L. is a cofounder of Prosetta Biosciences, and J.C.R. is a consultant for Prosetta Biosciences. V.R.L. is a cofounder, CEO, and CTO of Prosetta Biosciences.
Figures


References
-
- UNAIDS Global HIV & AIDS Statistics—2020 Fact Sheet. [(accessed on 19 January 2021)];2020 Available online: https://www.unaids.org/en/resources/fact-sheet.
-
- Boender T.S., Sigaloff K.C., McMahon J.H., Kiertiburanakul S., Jordan M.R., Barcarolo J., Ford N., Rinke de Wit T.F., Bertagnolio S. Long-term Virological Outcomes of First-Line Antiretroviral Therapy for HIV-1 in Low- and Middle-Income Countries: A Systematic Review and Meta-analysis. Clin. Infect. Dis. 2015;61:1453–1461. doi: 10.1093/cid/civ556. - DOI - PMC - PubMed
-
- Gregson J., Kaleebu P., Marconi V.C., van Vuuren C., Ndembi N., Hamers R.L., Kanki P., Hoffmann C.J., Lockman S., Pillay D., et al. Occult HIV-1 drug resistance to thymidine analogues following failure of first-line tenofovir combined with a cytosine analogue and nevirapine or efavirenz in sub Saharan Africa: A retrospective multi-centre cohort study. Lancet Infect. Dis. 2017;17:296–304. doi: 10.1016/S1473-3099(16)30469-8. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

