Actin-Resistant DNase1L2 as a Potential Therapeutics for CF Lung Disease
- PMID: 33802146
- PMCID: PMC8002113
- DOI: 10.3390/biom11030410
Actin-Resistant DNase1L2 as a Potential Therapeutics for CF Lung Disease
Abstract
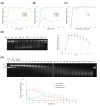
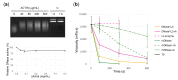
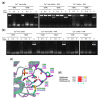
In cystic fibrosis (CF), the accumulation of viscous lung secretions rich in DNA and actin is a major cause of chronic inflammation and recurrent infections leading to airway obstruction. Mucolytic therapy based on recombinant human DNase1 reduces CF mucus viscosity and promotes airway clearance. However, the marked susceptibility to actin inhibition of this enzyme prompts the research of alternative treatments that could overcome this limitation. Within the human DNase repertoire, DNase1L2 is ideally suited for this purpose because it exhibits metal-dependent endonuclease activity on plasmid DNA in a broad range of pH with acidic optimum and is minimally inhibited by actin. When tested on CF artificial mucus enriched with actin, submicromolar concentrations of DNase1L2 reduces mucus viscosity by 50% in a few seconds. Inspection of superimposed model structures of DNase1 and DNase1L2 highlights differences at the actin-binding interface that justify the increased resistance of DNase1L2 toward actin inhibition. Furthermore, a PEGylated form of the enzyme with preserved enzymatic activity was obtained, showing interesting results in terms of activity. This work represents an effort toward the exploitation of natural DNase variants as promising alternatives to DNase1 for the treatment of CF lung disease.
Keywords: PEGylation; Pichia pastoris; cystic fibrosis; endonuclease; enzyme therapeutics; mucolytics.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures



References
-
- Lethem M.I., James S.L., Marriott C., Burke J.F. The origin of DNA associated with mucus glycoproteins in cystic fibrosis sputum. Eur. Respir. J. 1990;3:19–23. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

