LncRNAs in Cardiomyocyte Maturation: New Window for Cardiac Regenerative Medicine
- PMID: 33802186
- PMCID: PMC8005985
- DOI: 10.3390/ncrna7010020
LncRNAs in Cardiomyocyte Maturation: New Window for Cardiac Regenerative Medicine
Abstract
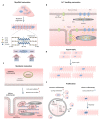
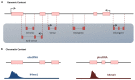
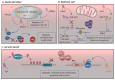
Cardiomyocyte (CM) maturation, which is characterized by structural, functional, and metabolic specializations, is the last phase of CM development that prepares the cells for efficient and forceful contraction throughout life. Over the past decades, CM maturation has gained increased attention due to the fact that pluripotent stem cell-derived CMs are structurally, transcriptionally, and functionally immature and embryonic-like, which causes a defect in cell replacement therapy. The current challenge is to discover and understand the molecular mechanisms, which control the CM maturation process. Currently, emerging shreds of evidence emphasize the role of long noncoding RNAs (lncRNAs) in regulating different aspects of CM maturation, including myofibril maturation, electrophysiology, and Ca2+ handling maturation, metabolic maturation and proliferation to hypertrophy transition. Here, we describe the structural and functional characteristics of mature CMs. Furthermore, this review highlights the lncRNAs as crucial regulators of different aspects in CM maturation, which have the potential to be used for mature CM production. With the current advances in oligonucleotide delivery; lncRNAs may serve as putative therapeutic targets to produce highly mature CMs for research and regenerative medicine.
Keywords: cardiomyocyte maturation; lncRNAs; myofibril maturation; regenerative medicine.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures


Similar articles
-
Cardiomyocyte Maturation: New Phase in Development.Circ Res. 2020 Apr 10;126(8):1086-1106. doi: 10.1161/CIRCRESAHA.119.315862. Epub 2020 Apr 9. Circ Res. 2020. PMID: 32271675 Free PMC article. Review.
-
Regulation of cardiomyocyte maturation during critical perinatal window.J Physiol. 2020 Jul;598(14):2941-2956. doi: 10.1113/JP276754. Epub 2019 Jan 15. J Physiol. 2020. PMID: 30571853 Free PMC article. Review.
-
Targeting HIF-1α in combination with PPARα activation and postnatal factors promotes the metabolic maturation of human induced pluripotent stem cell-derived cardiomyocytes.J Mol Cell Cardiol. 2019 Jul;132:120-135. doi: 10.1016/j.yjmcc.2019.05.003. Epub 2019 May 11. J Mol Cell Cardiol. 2019. PMID: 31082397 Free PMC article.
-
Learn from Your Elders: Developmental Biology Lessons to Guide Maturation of Stem Cell-Derived Cardiomyocytes.Pediatr Cardiol. 2019 Oct;40(7):1367-1387. doi: 10.1007/s00246-019-02165-5. Epub 2019 Aug 6. Pediatr Cardiol. 2019. PMID: 31388700 Free PMC article. Review.
-
Metabolic environment in vivo as a blueprint for differentiation and maturation of human stem cell-derived cardiomyocytes.Biochim Biophys Acta Mol Basis Dis. 2020 Oct 1;1866(10):165881. doi: 10.1016/j.bbadis.2020.165881. Epub 2020 Jun 17. Biochim Biophys Acta Mol Basis Dis. 2020. PMID: 32562698 Review.
Cited by
-
Heart regeneration: 20 years of progress and renewed optimism.Dev Cell. 2022 Feb 28;57(4):424-439. doi: 10.1016/j.devcel.2022.01.012. Dev Cell. 2022. PMID: 35231426 Free PMC article. Review.
-
Non-Coding RNAs in Stem Cell Regulation and Cardiac Regeneration: Current Problems and Future Perspectives.Int J Mol Sci. 2021 Aug 25;22(17):9160. doi: 10.3390/ijms22179160. Int J Mol Sci. 2021. PMID: 34502068 Free PMC article. Review.
-
Long Non-Coding RNAs in Cardiovascular Diseases: Potential Function as Biomarkers and Therapeutic Targets of Exercise Training.Noncoding RNA. 2021 Oct 11;7(4):65. doi: 10.3390/ncrna7040065. Noncoding RNA. 2021. PMID: 34698215 Free PMC article. Review.
-
Non-Coding RNAs in the Therapeutic Landscape of Pathological Cardiac Hypertrophy.Cells. 2022 May 31;11(11):1805. doi: 10.3390/cells11111805. Cells. 2022. PMID: 35681500 Free PMC article. Review.
-
Using human induced pluripotent stem cell-derived cardiomyocytes to understand the mechanisms driving cardiomyocyte maturation.Front Cardiovasc Med. 2022 Aug 12;9:967659. doi: 10.3389/fcvm.2022.967659. eCollection 2022. Front Cardiovasc Med. 2022. PMID: 36061558 Free PMC article.
References
-
- Liu Y.-W., Chen B., Yang X., Fugate J.A., Kalucki F.A., Futakuchi-Tsuchida A., Couture L., Vogel K.W., Astley C.A., Baldessari A., et al. Human embryonic stem cell–derived cardiomyocytes restore function in infarcted hearts of non-human primates. Nat. Biotechnol. 2018;36:597–605. doi: 10.1038/nbt.4162. - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

