Digestive Inflammation: Role of Proteolytic Dysregulation
- PMID: 33802197
- PMCID: PMC7999743
- DOI: 10.3390/ijms22062817
Digestive Inflammation: Role of Proteolytic Dysregulation
Abstract
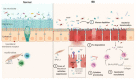
Dysregulation of the proteolytic balance is often associated with diseases. Serine proteases and matrix metalloproteases are involved in a multitude of biological processes and notably in the inflammatory response. Within the framework of digestive inflammation, several studies have stressed the role of serine proteases and matrix metalloproteases (MMPs) as key actors in its pathogenesis and pointed to the unbalance between these proteases and their respective inhibitors. Substantial efforts have been made in developing new inhibitors, some of which have reached clinical trial phases, notwithstanding that unwanted side effects remain a major issue. However, studies on the proteolytic imbalance and inhibitors conception are directed toward host serine/MMPs proteases revealing a hitherto overlooked factor, the potential contribution of their bacterial counterpart. In this review, we highlight the role of proteolytic imbalance in human digestive inflammation focusing on serine proteases and MMPs and their respective inhibitors considering both host and bacterial origin.
Keywords: digestive inflammation; gut microbiota; holobiont; protease; serpin.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

