Synthesis and Characterization of Exopolysaccharide Encapsulated PCL/Gelatin Skin Substitute for Full-Thickness Wound Regeneration
- PMID: 33802198
- PMCID: PMC8000589
- DOI: 10.3390/polym13060854
Synthesis and Characterization of Exopolysaccharide Encapsulated PCL/Gelatin Skin Substitute for Full-Thickness Wound Regeneration
Abstract
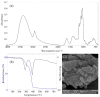
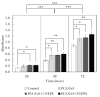
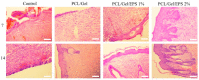
Loss of skin integrity can lead to serious problems and even death. In this study, for the first time, the effect of exopolysaccharide (EPS) produced by cold-adapted yeast R. mucilaginosa sp. GUMS16 on a full-thickness wound in rats was evaluated. The GUMS16 strain's EPS was precipitated by adding cold ethanol and then lyophilized. Afterward, the EPS with polycaprolactone (PCL) and gelatin was fabricated into nanofibers with two single-needle and double-needle procedures. The rats' full-thickness wounds were treated with nanofibers and Hematoxylin and eosin (H&E) and Masson's Trichrome staining was done for studying the wound healing in rats. Obtained results from SEM, DLS, FTIR, and TGA showed that EPS has a carbohydrate chemical structure with an average diameter of 40 nm. Cell viability assessments showed that the 2% EPS loaded sample exhibits the highest cell activity. Moreover, in vivo implantation of nanofiber webs on the full-thickness wound on rat models displayed a faster healing rate when EPS was loaded into a nanofiber. These results suggest that the produced EPS can be used for skin tissue engineering applications.
Keywords: exopolysaccharide; nanofiber; tissue regeneration; wound dressing.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures





Similar articles
-
Fabrication and characterization of biaxially electrospun collagen/alginate nanofibers, improved with Rhodotorula mucilaginosa sp. GUMS16 produced exopolysaccharides for wound healing applications.Int J Biol Macromol. 2022 Jan 31;196:194-203. doi: 10.1016/j.ijbiomac.2021.11.132. Epub 2021 Nov 28. Int J Biol Macromol. 2022. PMID: 34852259
-
Bioactive antibacterial bilayer PCL/gelatin nanofibrous scaffold promotes full-thickness wound healing.Int J Pharm. 2020 Jun 15;583:119413. doi: 10.1016/j.ijpharm.2020.119413. Epub 2020 May 7. Int J Pharm. 2020. PMID: 32389791
-
Burn wound healing using adipose-derived mesenchymal stem cells and manganese nanoparticles in polycaprolactone/gelatin electrospun nanofibers in rats.Bioimpacts. 2024;14(5):30193. doi: 10.34172/bi.2024.30193. Epub 2024 Jan 21. Bioimpacts. 2024. PMID: 39296800 Free PMC article.
-
Biomimetic PCL-gelatin based nanofibers loaded with ciprofloxacin hydrochloride and quercetin: A potential antibacterial and anti-oxidant dressing material for accelerated healing of a full thickness wound.Int J Pharm. 2019 Aug 15;567:118480. doi: 10.1016/j.ijpharm.2019.118480. Epub 2019 Jun 28. Int J Pharm. 2019. PMID: 31255776
-
Controlled release of lawsone from polycaprolactone/gelatin electrospun nano fibers for skin tissue regeneration.Int J Biol Macromol. 2019 Mar 1;124:478-491. doi: 10.1016/j.ijbiomac.2018.11.237. Epub 2018 Nov 27. Int J Biol Macromol. 2019. PMID: 30500508
Cited by
-
Exploring fungal bioemulsifiers: insights into chemical composition, microbial sources, and cross-field applications.World J Microbiol Biotechnol. 2024 Mar 7;40(4):127. doi: 10.1007/s11274-024-03883-6. World J Microbiol Biotechnol. 2024. PMID: 38451356 Review.
-
Fabrication and characterization of bilayer scaffolds made of decellularized dermis/nanofibrous collagen for healing of full-thickness wounds.Drug Deliv Transl Res. 2023 Jun;13(6):1766-1779. doi: 10.1007/s13346-023-01292-0. Epub 2023 Jan 26. Drug Deliv Transl Res. 2023. PMID: 36701113
-
Preparation and Investigation of Cellulose Acetate/Gelatin Janus Nanofiber Wound Dressings Loaded with Zinc Oxide or Curcumin for Enhanced Antimicrobial Activity.Membranes (Basel). 2024 Apr 23;14(5):95. doi: 10.3390/membranes14050095. Membranes (Basel). 2024. PMID: 38786930 Free PMC article.
-
Nanomaterials in Skin Regeneration and Rejuvenation.Int J Mol Sci. 2021 Jun 30;22(13):7095. doi: 10.3390/ijms22137095. Int J Mol Sci. 2021. PMID: 34209468 Free PMC article. Review.
-
Recent Advances in Functional Polymer Materials for Energy, Water, and Biomedical Applications: A Review.Polymers (Basel). 2021 Dec 10;13(24):4327. doi: 10.3390/polym13244327. Polymers (Basel). 2021. PMID: 34960878 Free PMC article. Review.
References
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

