A Placebo-Controlled, Pseudo-Randomized, Crossover Trial of Botanical Agents for Gulf War Illness: Curcumin (Curcuma longa), Boswellia (Boswellia serrata), and French Maritime Pine Bark (Pinus pinaster)
- PMID: 33802272
- PMCID: PMC7967595
- DOI: 10.3390/ijerph18052468
A Placebo-Controlled, Pseudo-Randomized, Crossover Trial of Botanical Agents for Gulf War Illness: Curcumin (Curcuma longa), Boswellia (Boswellia serrata), and French Maritime Pine Bark (Pinus pinaster)
Abstract
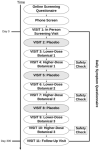
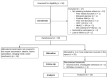
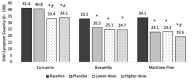
This report is part of a larger study designed to rapidly and efficiently screen potential treatments for Gulf War Illness (GWI) by testing nine different botanicals. In this placebo-controlled, pseudo-randomized, crossover clinical trial of 20 men with GWI, we tested three botanical agents with putative peripheral and central anti-inflammatory actions: curcumin (Curcuma longa), boswellia (Boswellia serrata), and French maritime pine bark extract (Pinus pinaster). Participants completed 30 +/- 3 days of baseline symptom reports, followed by 30 +/- 3 days of placebo, 30 +/- 3 days of lower-dose botanical, and 30 +/- 3 days of higher-dose botanical. Participants then repeated the process with a new botanical until completing up to three botanical cycles. Data were analyzed using linear mixed models. Curcumin reduced GWI symptom severity significantly more than placebo at both the lower (p < 0.0001) and higher (p = 0.0003) dosages. Boswellia was not more effective than placebo at reducing GWI symptoms at either the lower (p = 0.726) or higher (p = 0.869) dosages. Maritime pine was not more effective than placebo at the lower dosage (p = 0.954) but was more effective than placebo at the higher dosage (p = 0.006). This study provides preliminary evidence that curcumin and maritime pine may help alleviate symptoms of GWI. As a screening study, a final determination of the efficacy of these compounds for all individuals with GWI cannot be made, and further studies will need to be conducted to determine strength and durability of effects, as well as optimal dosage. These results suggest that GWI may, at least in part, involve systemic inflammatory processes. This trial was registered on ClinicalTrials.gov (NCT02909686) on 13 September 2016.
Keywords: boswellia; curcumin; maritime pine.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- White R.F., Steele L., O’Callaghan J.P., Sullivan K., Binns J.H., Golomb B.A., Bloom F.E., Bunker J.A., Crawford F., Graves J.C., et al. Recent research on Gulf War illness and other health problems in veterans of the 1991 Gulf War: Effects of toxicant exposures during deployment. Cortex A J. Devoted Study Nerv. Syst. Behav. 2016;74:449–475. doi: 10.1016/j.cortex.2015.08.022. - DOI - PMC - PubMed
-
- Khaiboullina S.F., DeMeirleir K.L., Rawat S., Berk G.S., Gaynor-Berk R.S., Mijatovic T., Blatt N., Rizvanov A.A., Young S.G., Lombardi V.C. Cytokine expression provides clues to the pathophysiology of Gulf War illness and myalgic encephalomyelitis. Cytokine. 2015;72:1–8. doi: 10.1016/j.cyto.2014.11.019. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

